Chloroquine resistance evolution in Plasmodium falciparum is mediated by the putative amino acid transporter AAT1
- PMID: 37169919
- PMCID: PMC10322710
- DOI: 10.1038/s41564-023-01377-z
Chloroquine resistance evolution in Plasmodium falciparum is mediated by the putative amino acid transporter AAT1
Abstract
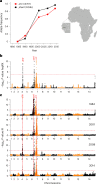
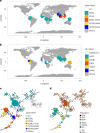
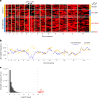
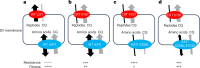
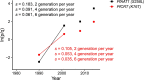
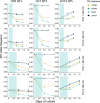
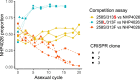
Malaria parasites break down host haemoglobin into peptides and amino acids in the digestive vacuole for export to the parasite cytoplasm for growth: interrupting this process is central to the mode of action of several antimalarial drugs. Mutations in the chloroquine (CQ) resistance transporter, pfcrt, located in the digestive vacuole membrane, confer CQ resistance in Plasmodium falciparum, and typically also affect parasite fitness. However, the role of other parasite loci in the evolution of CQ resistance is unclear. Here we use a combination of population genomics, genetic crosses and gene editing to demonstrate that a second vacuolar transporter plays a key role in both resistance and compensatory evolution. Longitudinal genomic analyses of the Gambian parasites revealed temporal signatures of selection on a putative amino acid transporter (pfaat1) variant S258L, which increased from 0% to 97% in frequency between 1984 and 2014 in parallel with the pfcrt1 K76T variant. Parasite genetic crosses then identified a chromosome 6 quantitative trait locus containing pfaat1 that is selected by CQ treatment. Gene editing demonstrated that pfaat1 S258L potentiates CQ resistance but at a cost of reduced fitness, while pfaat1 F313S, a common southeast Asian polymorphism, reduces CQ resistance while restoring fitness. Our analyses reveal hidden complexity in CQ resistance evolution, suggesting that pfaat1 may underlie regional differences in the dynamics of resistance evolution, and modulate parasite resistance or fitness by manipulating the balance between both amino acid and drug transport.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









Comment in
-
Two transporters enable chloroquine resistance in malaria.Nat Microbiol. 2023 Jul;8(7):1193-1194. doi: 10.1038/s41564-023-01414-x. Nat Microbiol. 2023. PMID: 37308594 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- MR/S009760/1/MRC_/Medical Research Council/United Kingdom
- C06 RR013556/RR/NCRR NIH HHS/United States
- R37 AI048071/AI/NIAID NIH HHS/United States
- P01 AI127338/AI/NIAID NIH HHS/United States
- 220221 /WT_/Wellcome Trust/United Kingdom
- G1100123/MRC_/Medical Research Council/United Kingdom
- MC_EX_MR/K02440X/1/MRC_/Medical Research Council/United Kingdom
- 206194 /WT_/Wellcome Trust/United Kingdom
- MC_EX_MR/J002364/1/MRC_/Medical Research Council/United Kingdom
- BB/M022161/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 204911 /WT_/Wellcome Trust/United Kingdom
- R01 AI048071/AI/NIAID NIH HHS/United States
- P51 OD011133/OD/NIH HHS/United States
- P30 CA054174/CA/NCI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources

