HSPB7 oppositely regulates human mesenchymal stromal cell-derived osteogenesis and adipogenesis
- PMID: 37170285
- PMCID: PMC10173662
- DOI: 10.1186/s13287-023-03361-0
HSPB7 oppositely regulates human mesenchymal stromal cell-derived osteogenesis and adipogenesis
Abstract
Background: Recent evidence suggests that accumulation of marrow adipose tissue induced by aberrant lineage allocation of bone marrow-derived mesenchymal stromal cells (BMSCs) contributes to the pathophysiologic processes of osteoporosis. Although master regulators of lineage commitment have been well documented, molecular switches between osteogenesis and adipogenesis are largely unknown.
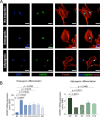
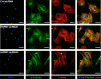
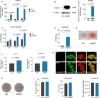
Methods: HSPB7 gene expression during osteogenic and adipogenic differentiation of BMSCs was evaluated by qPCR and Western blot analyses. Lentiviral-mediated knockdown or overexpression of HSPB7 and its deletion constructs were used to assess its function. The organization of cytoskeleton was examined by immunofluorescent staining. ALP activity, calcium assay, Alizarin Red S staining and Oil Red O staining were performed in vitro during osteoblast or adipocyte differentiation. SB431542 and Activin A antibody were used to identify the mechanism of Activin A in the regulation of osteogenic differentiation in BMSCs.
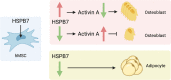
Results: In this study, we identified HSPB7 capable of oppositely regulating osteogenic and adipogenic differentiation of BMSCs. HSPB7 silencing promoted adipogenesis while reducing osteogenic differentiation and mineralization. Conversely, overexpression of HSPB7 strongly enhanced osteogenesis, but no effect was observed on adipogenic differentiation. Deletion of the N-terminal or C-terminal domain of HSPB7 led to decreased osteoblastic potency and mineralization. Mechanistically, our data showed that Activin A is a downstream target participating in HSPB7 knockdown-mediated osteogenic inhibition.
Conclusions: Our findings suggest that HSPB7 plays a positive role in driving osteoblastic differentiation, and with the capability in maintaining the osteo-adipogenesis balance. It holds great promise as a potential therapeutic target in the treatment of bone metabolic diseases.
Keywords: Activin A; Adipogenic differentiation; Bone marrow-derived mesenchymal stromal cells; HSPB7; Lineage commitment; Osteogenic differentiation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

