Efficacy and safety of molnupiravir for the treatment of SARS-CoV-2 infection: a systematic review and meta-analysis
- PMID: 37170886
- PMCID: PMC10320168
- DOI: 10.1093/jac/dkad132
Efficacy and safety of molnupiravir for the treatment of SARS-CoV-2 infection: a systematic review and meta-analysis
Abstract
Background: The role of molnupiravir for coronavirus disease 2019 (COVID-19) treatment is unclear.
Methods: We conducted a systematic review until 1 November 2022 searching for randomized controlled trials (RCTs) involving COVID-19 patients comparing molnupiravir [±standard of care (SoC)] versus SoC and/or placebo. Data were pooled in random-effects meta-analyses. Certainty of evidence was assessed according to the Grading of Recommendations, Assessment, Development and Evaluations approach.
Results: Nine RCTs were identified, eight investigated outpatients (29 254 participants) and one inpatients (304 participants). Compared with placebo/SoC, molnupiravir does not reduce mortality [risk ratio (RR) 0.27, 95% CI 0.07-1.02, high-certainty evidence] and probably does not reduce the risk for 'hospitalization or death' (RR 0.81, 95% CI 0.55-1.20, moderate-certainty evidence) by Day 28 in COVID-19 outpatients. We are uncertain whether molnupiravir increases symptom resolution by Day 14 (RR 1.20, 95% CI 1.02-1.41, very-low-certainty evidence) but it may make no difference by Day 28 (RR 1.05, 95% CI 0.92-1.19, low-certainty evidence). In inpatients, molnupiravir may increase mortality by Day 28 compared with placebo (RR 3.78, 95% CI 0.50-28.82, low-certainty evidence). There is little to no difference in serious adverse and adverse events during the study period in COVID-19 inpatients/outpatients treated with molnupiravir compared with placebo/SoC (moderate- to high-certainty evidence).
Conclusions: In a predominantly immunized population of COVID-19 outpatients, molnupiravir has no effect on mortality, probably none on 'hospitalization or death' and effects on symptom resolution are uncertain. Molnupiravir was safe during the study period in outpatients although a potential increase in inpatient mortality requires careful monitoring in ongoing clinical research. Our analysis does not support routine use of molnupiravir for COVID-19 treatment in immunocompetent individuals.
© The Author(s) 2023. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures

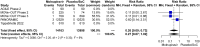
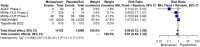

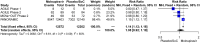


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

