Fluorinated carbohydrates for 18F-positron emission tomography (PET)
- PMID: 37171037
- PMCID: PMC10243284
- DOI: 10.1039/d3cs00037k
Fluorinated carbohydrates for 18F-positron emission tomography (PET)
Abstract
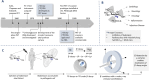
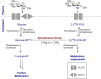
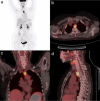
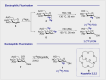
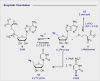
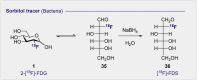
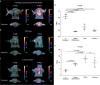
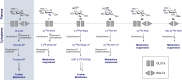
Carbohydrate diversity is foundational in the molecular literacy that regulates cellular function and communication. Consequently, delineating and leveraging this structure-function interplay continues to be a core research objective in the development of candidates for biomedical diagnostics. A totemic example is the ubiquity of 2-deoxy-2-[18F]-fluoro-D-glucose (2-[18F]-FDG) as a radiotracer for positron emission tomography (PET), in which metabolic trapping is harnessed. Building on this clinical success, more complex sugars with unique selectivities are gaining momentum in molecular recognition and personalised medicine: this reflects the opportunities that carbohydrate-specific targeting affords in a broader sense. In this Tutorial Review, key milestones in the development of 2-[18F]-FDG and related glycan-based radiotracers for PET are described, with their diagnostic functions, to assist in navigating this rapidly expanding field of interdisciplinary research.
Conflict of interest statement
There are no conflicts to declare.
Figures









Similar articles
-
Evaluation of [18F]FDG/[18F]FLT/[18F]FMISO-based micro-positron emission tomography in detection of liver metastasis in human colorectal cancer.Nucl Med Biol. 2019 May-Jun;72-73:36-44. doi: 10.1016/j.nucmedbio.2019.07.004. Epub 2019 Jul 11. Nucl Med Biol. 2019. PMID: 31330410
-
Update on advances in molecular PET in urological oncology.Jpn J Radiol. 2016 Jul;34(7):470-85. doi: 10.1007/s11604-016-0553-3. Epub 2016 May 24. Jpn J Radiol. 2016. PMID: 27222021 Free PMC article. Review.
-
The role of (18F)-fluoro-D-glucose positron emission tomography/computed tomography in the surveillance of abnormal myocardial energy metabolism and cardiac dysfunction in a rat model of cardiopulmonary resuscitation.Diagn Interv Radiol. 2023 May 31;29(3):548-554. doi: 10.4274/dir.2023.221932. Epub 2023 May 8. Diagn Interv Radiol. 2023. PMID: 37154799 Free PMC article.
-
[The value of 18F-fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG PET) in diagnosis of neoplastic diseases].Med Clin (Barc). 2005 Feb 19;124(6):229-36. doi: 10.1157/13071769. Med Clin (Barc). 2005. PMID: 15737307 Review. Spanish.
-
Role of ¹⁸F 2-fluoro-2-deoxyglucose positron emission tomography in upper gastrointestinal malignancies.World J Gastroenterol. 2011 Dec 14;17(46):5059-74. doi: 10.3748/wjg.v17.i46.5059. World J Gastroenterol. 2011. PMID: 22171140 Free PMC article. Review.
Cited by
-
Unravelling structure-function interactions between fluorinated heparan sulfate mimetics and signaling proteins.RSC Chem Biol. 2025 Jul 10. doi: 10.1039/d5cb00174a. Online ahead of print. RSC Chem Biol. 2025. PMID: 40755491 Free PMC article.
-
Probing the Origin of Affinity in the GM1-Cholera Toxin Complex through Site-Selective Editing with Fluorine.ACS Cent Sci. 2024 Jul 12;10(8):1481-1489. doi: 10.1021/acscentsci.4c00622. eCollection 2024 Aug 28. ACS Cent Sci. 2024. PMID: 39220706 Free PMC article.
-
Positron scattering from structurally related biomolecules.RSC Adv. 2024 Jan 3;14(2):1397-1406. doi: 10.1039/d3ra06227a. eCollection 2024 Jan 2. RSC Adv. 2024. PMID: 38174274 Free PMC article.
-
Expediting Glycospace Exploration: Therapeutic Glycans via Automated Synthesis.Angew Chem Int Ed Engl. 2025 Mar 24;64(13):e202422766. doi: 10.1002/anie.202422766. Epub 2025 Feb 21. Angew Chem Int Ed Engl. 2025. PMID: 39936247 Free PMC article. Review.
-
Radioactive Molecules 2021-2022.Molecules. 2024 Jan 4;29(1):265. doi: 10.3390/molecules29010265. Molecules. 2024. PMID: 38202848 Free PMC article.
References
-
- Gallagher B. M. Ansari A. Atkins H. Casella V. Christman D. R. Fowler J. S. Ido T. MacGregor R. R. Som P. Wan C. N. Wolf A. P. Kuhl D. E. Reivich M. J. Nucl. Med. 1977;18:990–996. - PubMed
-
- Ido T. Wan C. N. Casella V. Fowler J. S. Wolf A. P. Reivich M. Kuhl D. E. J. Label. Compd. Radiopharm. 1978;14:175–183. doi: 10.1002/jlcr.2580140204. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

