Nesprin-1: novel regulator of striated muscle nuclear positioning and mechanotransduction
- PMID: 37171063
- PMCID: PMC10317153
- DOI: 10.1042/BST20221541
Nesprin-1: novel regulator of striated muscle nuclear positioning and mechanotransduction
Abstract
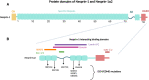
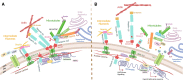
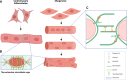
Nesprins (nuclear envelope spectrin repeat proteins) are multi-isomeric scaffolding proteins. Giant nesprin-1 and -2 localise to the outer nuclear membrane, interact with SUN (Sad1p/UNC-84) domain-containing proteins at the inner nuclear membrane to form the LInker of Nucleoskeleton and Cytoskeleton (LINC) complex, which, in association with lamin A/C and emerin, mechanically couples the nucleus to the cytoskeleton. Despite ubiquitous expression of nesprin giant isoforms, pathogenic mutations in nesprin-1 and -2 are associated with tissue-specific disorders, particularly related to striated muscle such as dilated cardiomyopathy and Emery-Dreifuss muscular dystrophy. Recent evidence suggests this muscle-specificity might be attributable in part, to the small muscle specific isoform, nesprin-1α2, which has a novel role in striated muscle function. Our current understanding of muscle-specific functions of nesprin-1 and its isoforms will be summarised in this review to provide insight into potential pathological mechanisms of nesprin-related muscle disease and may inform potential targets of therapeutic modulation.
Keywords: DCM and EDMD; mechanotransduction; microtubule; nesprin; nuclear envelope LINC complex; nuclear positioning.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
Similar articles
-
Nesprin-1/2: roles in nuclear envelope organisation, myogenesis and muscle disease.Biochem Soc Trans. 2018 Apr 17;46(2):311-320. doi: 10.1042/BST20170149. Epub 2018 Feb 27. Biochem Soc Trans. 2018. PMID: 29487227 Review.
-
Mouse models of nesprin-related diseases.Biochem Soc Trans. 2018 Jun 19;46(3):669-681. doi: 10.1042/BST20180085. Epub 2018 May 21. Biochem Soc Trans. 2018. PMID: 29784648 Review.
-
Novel nesprin-1 mutations associated with dilated cardiomyopathy cause nuclear envelope disruption and defects in myogenesis.Hum Mol Genet. 2017 Jun 15;26(12):2258-2276. doi: 10.1093/hmg/ddx116. Hum Mol Genet. 2017. PMID: 28398466 Free PMC article.
-
Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity.Hum Mol Genet. 2007 Dec 1;16(23):2816-33. doi: 10.1093/hmg/ddm238. Epub 2007 Aug 29. Hum Mol Genet. 2007. PMID: 17761684
-
Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle.J Cell Sci. 2005 Feb 15;118(Pt 4):673-87. doi: 10.1242/jcs.01642. Epub 2005 Jan 25. J Cell Sci. 2005. PMID: 15671068
Cited by
-
The Drosophila Nesprin-1 homolog MSP300 is required for muscle autophagy and proteostasis.J Cell Sci. 2024 Jun 1;137(11):jcs262096. doi: 10.1242/jcs.262096. Epub 2024 Jun 10. J Cell Sci. 2024. PMID: 38757366 Free PMC article.
-
Mechanical Forces, Nucleus, Chromosomes, and Chromatin.Biomolecules. 2025 Mar 1;15(3):354. doi: 10.3390/biom15030354. Biomolecules. 2025. PMID: 40149890 Free PMC article. Review.
-
WAVE2 Is a Vital Regulator in Myogenic Differentiation of Progenitor Cells through the Mechanosensitive MRTFA-SRF Axis.Cells. 2023 Dec 20;13(1):9. doi: 10.3390/cells13010009. Cells. 2023. PMID: 38201213 Free PMC article.
-
Acute microtubule changes linked to DMD pathology are insufficient to impair contractile function or enhance contraction-induced injury in healthy muscle.bioRxiv [Preprint]. 2024 Jun 23:2024.06.19.599775. doi: 10.1101/2024.06.19.599775. bioRxiv. 2024. PMID: 38948772 Free PMC article. Preprint.
-
Enhanced cell viscosity: A new phenotype associated with lamin A/C alterations.iScience. 2023 Aug 25;26(10):107714. doi: 10.1016/j.isci.2023.107714. eCollection 2023 Oct 20. iScience. 2023. PMID: 37701573 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

