A randomized comparison of CPX-351 and FLAG-Ida in adverse karyotype AML and high-risk MDS: the UK NCRI AML19 trial
- PMID: 37171402
- PMCID: PMC10425682
- DOI: 10.1182/bloodadvances.2023010276
A randomized comparison of CPX-351 and FLAG-Ida in adverse karyotype AML and high-risk MDS: the UK NCRI AML19 trial
Abstract
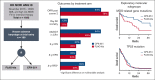
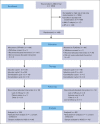
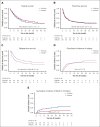
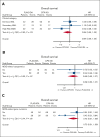
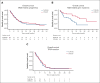
Liposomal daunorubicin and cytarabine (CPX-351) improved overall survival (OS) compared with 7+3 chemotherapy in older patients with secondary acute myeloid leukemia (AML); to date, there have been no randomized studies in younger patients. The high-risk cohort of the UK NCRI AML19 trial (ISRCTN78449203) compared CPX-351 with FLAG-Ida in younger adults with newly diagnosed adverse cytogenetic AML or high-risk myelodysplastic syndromes (MDS). A total of 189 patients were randomized (median age, 56 years). Per clinical criteria, 49% of patients had de novo AML, 20% had secondary AML, and 30% had high-risk MDS. MDS-related cytogenetics were present in 73% of the patients, with a complex karyotype in 49%. TP53 was the most common mutated gene, in 43%. Myelodysplasia-related gene mutations were present in 75 (44%) patients. The overall response rate (CR + CRi) after course 2 was 64% and 76% for CPX-351 and FLAG-Ida, respectively. There was no difference in OS (13.3 months vs 11.4 months) or event-free survival in multivariable analysis. However, relapse-free survival was significantly longer with CPX-351 (median 22.1 vs 8.35 months). There was no difference between the treatment arms in patients with clinically defined secondary AML or those with MDS-related cytogenetic abnormalities; however, an exploratory subgroup of patients with MDS-related gene mutations had significantly longer OS with CPX-351 (median 38.4 vs 16.3 months). In conclusion, the OS of younger patients with adverse risk AML/MDS was not significantly different between CPX-351 and FLAG-Ida.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.D. declares research funding from AbbVie and Amgen and consultancy with Astellas, Pfizer, Novartis, Jazz, BeiGene, Shattuck, and AvenCell. S.K. declares research funding from Novartis; speakers bureau with Astellas, Novartis and Jazz; consultancy with Servier and Bristol Myers Squibb. S.D.F. declares research funding from Jazz and Bristol Myers Squibb; speakers bureau with Jazz, Pfizer, and Novartis; and the advisory committee with Neogenomic. P.M. declares the honoraria and speakers bureau to Pfizer, Jazz, AbbVie, and Astellas. N.H.R. declares research funding from Jazz and Pfizer and honoraria from Pfizer, Servier, and Astellas. The remaining authors declare no competing financial interests.
Figures
References
-
- Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in aml: analysis of 1,612 patients entered into the MRC AML 10 Trial. Blood. 1998;92(7):2322–2333. - PubMed
-
- Hulegårdh E, Nilsson C, Lazarevic V, et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry. Am J Hematol. 2015;90(3):208–214. - PubMed
-
- Granfeldt Østgård LS, Medeiros BC, Sengeløv H, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641–3649. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

