Organic Electronic Platform for Real-Time Phenotypic Screening of Extracellular-Vesicle-Driven Breast Cancer Metastasis
- PMID: 37171457
- PMCID: PMC11468090
- DOI: 10.1002/adhm.202301194
Organic Electronic Platform for Real-Time Phenotypic Screening of Extracellular-Vesicle-Driven Breast Cancer Metastasis
Abstract
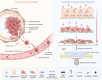
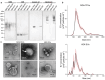
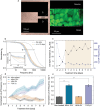
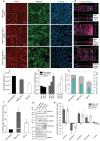
Tumor-derived extracellular vesicles (TEVs) induce the epithelial-to-mesenchymal transition (EMT) in nonmalignant cells to promote invasion and cancer metastasis, representing a novel therapeutic target in a field severely lacking in efficacious antimetastasis treatments. However, scalable technologies that allow continuous, multiparametric monitoring for identifying metastasis inhibitors are absent. Here, the development of a functional phenotypic screening platform based on organic electrochemical transistors (OECTs) for real-time, noninvasive monitoring of TEV-induced EMT and screening of antimetastatic drugs is reported. TEVs derived from the triple-negative breast cancer cell line MDA-MB-231 induce EMT in nonmalignant breast epithelial cells (MCF10A) over a nine-day period, recapitulating a model of invasive ductal carcinoma metastasis. Immunoblot analysis and immunofluorescence imaging confirm the EMT status of TEV-treated cells, while dual optical and electrical readouts of cell phenotype are obtained using OECTs. Further, heparin, a competitive inhibitor of cell surface receptors, is identified as an effective blocker of TEV-induced EMT. Together, these results demonstrate the utility of the platform for TEV-targeted drug discovery, allowing for facile modeling of the transient drug response using electrical measurements, and provide proof of concept that inhibitors of TEV function have potential as antimetastatic drug candidates.
Keywords: breast cancer metastasis; drug screening; exosomes; extracellular vesicles; organic electronics.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

