The predictive value of pretherapy [68Ga]Ga-DOTA-TATE PET and biomarkers in [177Lu]Lu-PRRT tumor dosimetry
- PMID: 37171633
- PMCID: PMC10981963
- DOI: 10.1007/s00259-023-06252-x
The predictive value of pretherapy [68Ga]Ga-DOTA-TATE PET and biomarkers in [177Lu]Lu-PRRT tumor dosimetry
Abstract
Purpose: Metastatic neuroendocrine tumors (NETs) overexpressing type 2 somatostatin receptors are the target for peptide receptor radionuclide therapy (PRRT) through the theragnostic pair of 68Ga/177Lu-DOTATATE. The main purpose of this study was to develop machine learning models to predict therapeutic tumor dose using pre therapy 68Ga -PET and clinicopathological biomarkers.
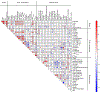
Methods: We retrospectively analyzed 90 segmented metastatic NETs from 25 patients (M14/F11, age 63.7 ± 9.5, range 38-76) treated by 177Lu-DOTATATE at our institute. Patients underwent both pretherapy [68Ga]Ga-DOTA-TATE PET/CT and four timepoints SPECT/CT at ~ 4, 24, 96, and 168 h post-177Lu-DOTATATE infusion. Tumors were segmented by a radiologist on baseline CT or MRI and transferred to co-registered PET/CT and SPECT/CT, and normal organs were segmented by deep learning-based method on CT of the PET and SPECT. The SUV metrics and tumor-to-normal tissue SUV ratios (SUV_TNRs) were calculated from 68Ga -PET at the contour-level. Posttherapy dosimetry was performed based on the co-registration of SPECT/CTs to generate time-integrated-activity, followed by an in-house Monte Carlo-based absorbed dose estimation. The correlation between delivered 177Lu Tumor absorbed dose and PET-derived metrics along with baseline clinicopathological biomarkers (such as Creatinine, Chromogranin A and prior therapies) were evaluated. Multiple interpretable machine-learning algorithms were developed to predict tumor dose using these pretherapy information. Model performance on a nested tenfold cross-validation was evaluated in terms of coefficient of determination (R2), mean-absolute-error (MAE), and mean-relative-absolute-error (MRAE).
Results: SUVmean showed a significant correlation (q-value < 0.05) with absorbed dose (Spearman ρ = 0.64), followed by TLSUVmean (SUVmean of total-lesion-burden) and SUVpeak (ρ = 0.45 and 0.41, respectively). The predictive value of PET-SUVmean in estimation of posttherapy absorbed dose was stronger compared to PET-SUVpeak, and SUV_TNRs in terms of univariate analysis (R2 = 0.28 vs. R2 ≤ 0.12). An optimal trivariate random forest model composed of SUVmean, TLSUVmean, and total liver SUVmean (normal and tumoral liver) provided the best performance in tumor dose prediction with R2 = 0.64, MAE = 0.73 Gy/GBq, and MRAE = 0.2.
Conclusion: Our preliminary results demonstrate the feasibility of using baseline PET images for prediction of absorbed dose prior to 177Lu-PRRT. Machine learning models combining multiple PET-based metrics performed better than using a single SUV value and using other investigated clinicopathological biomarkers. Developing such quantitative models forms the groundwork for the role of 68Ga -PET not only for the implementation of personalized treatment planning but also for patient stratification in the era of precision medicine.
Keywords: 177Lu-DOTATATE; Dosimetry; Machine learning; Theragnostic.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Figures




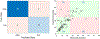
References
-
- Del Prete M, et al. Personalized (177)Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: initial results from the P-PRRT trial. Eur J Nucl Med Mol Imaging. 2019;46(3):728–42. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

