Heterologous Expression of Plantaricin 423 and Mundticin ST4SA in Saccharomyces cerevisiae
- PMID: 37171691
- PMCID: PMC11126478
- DOI: 10.1007/s12602-023-10082-6
Heterologous Expression of Plantaricin 423 and Mundticin ST4SA in Saccharomyces cerevisiae
Abstract
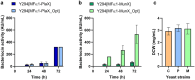
Antimicrobial peptides or bacteriocins are excellent candidates for alternative antimicrobials, but high manufacturing costs limit their applications. Recombinant gene expression offers the potential to produce these peptides more cost-effectively at a larger scale. Saccharomyces cerevisiae is a popular host for recombinant protein production, but with limited success reported on antimicrobial peptides. Individual recombinant S. cerevisiae strains were constructed to secrete two class IIa bacteriocins, plantaricin 423 (PlaX) and mundticin ST4SA (MunX). The native and codon-optimised variants of the plaA and munST4SA genes were cloned into episomal expression vectors containing either the S. cerevisiae alpha mating factor (MFα1) or the Trichoderma reesei xylanase 2 (XYNSEC) secretion signal sequences. The recombinant peptides retained their activity and stability, with the MFα1 secretion signal superior to the XYNSEC secretion signal for both bacteriocins. An eight-fold increase in activity against Listeria monocytogenes was observed for MunX after codon optimisation, but not for PlaX-producing strains. After HPLC-purification, the codon-optimised genes yielded 20.9 mg/L of MunX and 18.4 mg/L of PlaX, which displayed minimum inhibitory concentrations (MICs) of 108.52 nM and 1.18 µM, respectively, against L. monocytogenes. The yields represent a marked improvement relative to an Escherichia coli expression system previously reported for PlaX and MunX. The results demonstrated that S. cerevisiae is a promising host for recombinant bacteriocin production that requires a simple purification process, but the efficacy is sensitive to codon usage and secretion signals.
Keywords: Saccharomyces cerevisiae; Antimicrobial peptides; Class IIa bacteriocins; Heterologous expression.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Yi Y, Li P, Zhao F, Zhang T, Shan Y, Wang X, Liu B, Chen Y, Zhao X, Lü X. Current status and potentiality of class II bacteriocins from lactic acid bacteria: structure, mode of action and applications in the Food Industry. Trends Food Sci Technol. 2022;120:387–401. doi: 10.1016/j.tifs.2022.01.018. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

