Real-World Apremilast Use for Treatment of Plaque Psoriasis in Italy: Patient Perspective, Characteristics, and Clinical Outcomes from the DARWIN Study
- PMID: 37171752
- PMCID: PMC10175925
- DOI: 10.1007/s12325-023-02516-y
Real-World Apremilast Use for Treatment of Plaque Psoriasis in Italy: Patient Perspective, Characteristics, and Clinical Outcomes from the DARWIN Study
Abstract
Introduction: While several European studies have reported real-world apremilast use, patient-perceived benefits, and treatment satisfaction, local reimbursement criteria for apremilast vary and data from Italy are limited.
Methods: The cross-sectional DARWIN study enrolled consecutive patients who had initiated apremilast for plaque psoriasis 6 (± 1) months prior to enrolment at a single visit across 24 Italian dermatological sites. Disease severity was assessed using body surface area (BSA) and Physician Global Assessment (PGA). Patient-reported outcomes assessed 6 (± 1) months after apremilast initiation were Dermatology Life Quality Index (DLQI), Patient Benefit Index (PBI), and 9-item Treatment Satisfaction Questionnaire for Medication (TSQM-9).
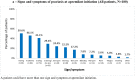
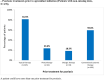
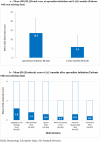
Results: Of 184 patients enrolled between July 2019 and January 2021, 180 were included in the analysis. At apremilast initiation, median (25th-75th percentile) time since psoriasis diagnosis was 8.6 (3.2-22.2) years; median BSA, 10.0% (5.0-16.0); mean (standard seviation, SD) DLQI total score, 13.5 (8.0). Over half (54.9%) of patients with available data reported psoriasis had a very or extremely large effect on their quality of life (QoL); half reported itching (50.6%) and/or special areas involvement (50.0%). Most (73.9%) had comorbidities and were biologic-naïve (81.5%). The most common reasons for initiating apremilast were lack of efficacy of previous treatment (56.7%) and contraindications to other treatments (44.4%). At 6 (± 1) months, most patients were continuing apremilast and/or reported a Global PBI score ≥ 1 (minimum clinical benefit) (86.1% and 90.0%, respectively); approximately half achieved BSA ≤ 3% and/or DLQI total score ≤ 5 (47.1% and 48.5%); 18.8% achieved PGA = 0; mean (SD) TSQM-9 global treatment satisfaction score was 59.0 (24.8). Apremilast was well tolerated; no new safety signals were identified.
Conclusions: Patients treated with apremilast for 6 months in Italian clinical practice reported improved QoL, clinically relevant improvements in symptoms, high treatment satisfaction, and high treatment persistence. Our data indicate apremilast is a valuable treatment option for moderate plaque psoriasis.
Study registration: ClinicalTrials.gov identifier, NCT04031027.
Keywords: Apremilast; Dermatology Life Quality Index; Italian real-world evidence; Patient Benefit Index; Patient perspective; Patient-reported outcomes; Plaque psoriasis; Psoriasis comorbidities; Quality of life; Treatment satisfaction.
© 2023. The Author(s).
Conflict of interest statement
Luca Bianchi served on advisory boards and received honoraria for lectures and research grants from Almirall, AbbVie, Leo Pharma, Amgen, Biogen, UCB, Eli Lilly, Janssen, Novartis, and Sanofi Genzyme. Gabriella Fabbrocini acted as speaker or consultant for AbbVie, Amgen, Eli Lilly, Janssen, Leo Pharma, Almirall, Novartis, and UCB. Paolo Gisondi served as consultant and/or speaker for AbbVie, Almirall, Amgen, Janssen, Leo-Pharma, Eli Lilly, Novartis, Pierre Fabre, Sandoz, Sanofi, UCB. Claudia Giofrè served as consultant for Janssen, Novartis, Leo-Pharma, Amgen, AbbVie, Sanofi and Lilly. Concetta Potenza has no conflict of interest to declare. Rossana Tiberio has no conflict of interest to declare. Claudio Marasca has no conflict of interest to declare. Emiliana Benincasa and Carmen M. A. Nuzzo are Amgen Inc. employees.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

