Making a good egg: human oocyte health, aging, and in vitro development
- PMID: 37171807
- PMCID: PMC10625843
- DOI: 10.1152/physrev.00032.2022
Making a good egg: human oocyte health, aging, and in vitro development
Abstract
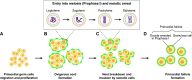
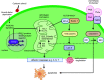
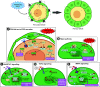
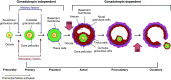
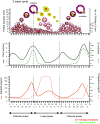
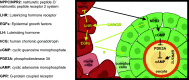
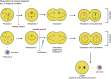
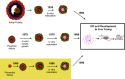
Mammalian eggs (oocytes) are formed during fetal life and establish associations with somatic cells to form primordial follicles that create a store of germ cells (the primordial pool). The size of this pool is influenced by key events during the formation of germ cells and by factors that influence the subsequent activation of follicle growth. These regulatory pathways must ensure that the reserve of oocytes within primordial follicles in humans lasts for up to 50 years, yet only approximately 0.1% will ever be ovulated with the rest undergoing degeneration. This review outlines the mechanisms and regulatory pathways that govern the processes of oocyte and follicle formation and later growth, within the ovarian stroma, through to ovulation with particular reference to human oocytes/follicles. In addition, the effects of aging on female reproductive capacity through changes in oocyte number and quality are emphasized, with both the cellular mechanisms and clinical implications discussed. Finally, the details of current developments in culture systems that support all stages of follicle growth to generate mature oocytes in vitro and emerging prospects for making new oocytes from stem cells are outlined.
Keywords: follicle culture; meiosis; oocyte maturation; ovary; reproductive aging; stem cells.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Harvey W. Exercitationes de generatione animalium. In: Du Gardanis. London: Du-Gardianis, 1651.
-
- Von Baer KE. De Ovi Mammalium et Hominis Genesi Epistolam ad Academiam Imperialem Scientiarum Petropolitanam dedit Carolus Ernestus a Baer. Leipzig, Germany: Leopold Voss, 1827.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

