Current and Optimal Practices in Childhood Asthma Monitoring Among Multiple International Stakeholders
- PMID: 37171821
- PMCID: PMC10182430
- DOI: 10.1001/jamanetworkopen.2023.13120
Current and Optimal Practices in Childhood Asthma Monitoring Among Multiple International Stakeholders
Abstract
Importance: Childhood asthma control largely depends on rigorous and regular monitoring. Although various clinical parameters, biomarkers, and patient-reported outcomes are helpful for monitoring purposes, there is no consensus on the minimum and/or optimal set of parameters and their relative priority.
Objective: To assess actual and perceived optimal childhood asthma monitoring practices used globally.
Design, setting, and participants: This international, multistakeholder survey study surveyed health care professionals and clinical academics with a professional interest in and exposure to childhood asthma between April 12 and September 3, 2021, to test for differences between the frequency that different techniques are actually used in practice vs optimal practice, between-group differences, and differences across medical settings and country economies.
Main outcomes and measures: Outcomes were frequency of duration of asthma monitoring visits as well as actual and perceived optimal use and importance of monitoring tools and domains.
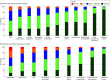
Results: A total of 1319 participants with expertise in childhood asthma from 88 countries completed the survey. Participants included 1228 health care professionals with a balanced distribution across different care settings (305 [22.7%] primary care, 401 [29.9%] secondary, and 522 [38.9%] tertiary care) and 91 researchers. Children with mild to moderate asthma attended regular monitoring visits at a median (IQR) of 5.0 (2.5-8.0) months, with visits lasting a median (IQR) of 25 (15-25) minutes, whereas severe asthma required more frequent visits (median [IQR], 2.5 [1.0-2.5] months; median [IQR] duration, 25 [25-35] minutes). Monitoring of symptoms and control, adherence, comorbidities, lung function, medication adverse effects, and allergy were considered to be very high or high priority by more than 75% of the respondents. Different patterns emerged when assessing differences between actual and perceived optimal use of monitoring tools. For some tools, current and optimal practices did not differ much (eg, spirometry), whereas in others, there was considerable space for improvement (eg, standardized control and adherence tests). The largest gap was observed for between-visit monitoring with electronic trackers, apps, and smart devices. Differences across country economies, care settings, and medical specialties were modest.
Conclusions and relevance: These survey results suggest that pediatric asthma monitoring is performed generally homogeneously worldwide, in most cases following evidence-based standards. Wider use of standardized instruments and the intensification of continuous between-visit monitoring, supported by electronic devices, is needed for further improvement of disease outcomes. The results of this survey, in conjunction with the available evidence base, can inform recommendations toward further optimization.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

