Androgen signalling in the ovaries and endometrium
- PMID: 37171897
- PMCID: PMC10663053
- DOI: 10.1093/molehr/gaad017
Androgen signalling in the ovaries and endometrium
Abstract
Currently, our understanding of hormonal regulation within the female reproductive system is largely based on our knowledge of estrogen and progesterone signalling. However, while the important functions of androgens in male physiology are well known, it is also recognized that androgens play critical roles in the female reproductive system. Further, androgen signalling is altered in a variety of gynaecological conditions, including endometriosis and polycystic ovary syndrome, indicative of regulatory roles in endometrial and ovarian function. Co-regulatory mechanisms exist between different androgens, estrogens, and progesterone, resulting in a complex network of steroid hormone interactions. Evidence from animal knockout studies, in vitro experiments, and human data indicate that androgen receptor expression is cell-specific and menstrual cycle stage-dependent, with important regulatory roles in the menstrual cycle, endometrial biology, and follicular development in the ovaries. This review will discuss the expression and co-regulatory interactions of androgen receptors, highlighting the complexity of the androgen signalling pathway in the endometrium and ovaries, and the synthesis of androgens from additional alternative pathways previously disregarded as male-specific. Moreover, it will illustrate the challenges faced when studying androgens in female biology, and the need for a more in-depth, integrative view of androgen metabolism and signalling in the female reproductive system.
Keywords: androgens; endometrium; estrogen; female; ovaries; progesterone; receptor; steroid hormones; testosterone.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
The authors declare no conflicts of interest.
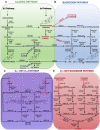
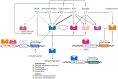
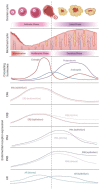
Figures
References
-
- Aflatounian A, Edwards MC, Paris VR, Bertoldo MJ, Desai R, Gilchrist RB, Ledger WL, Handelsman DJ, Walters KA.. Androgen signaling pathways driving reproductive and metabolic phenotypes in a PCOS mouse model. J Endocrinol 2020;245:381–395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

