TREM2 inhibition triggers antitumor cell activity of myeloid cells in glioblastoma
- PMID: 37172094
- PMCID: PMC10181199
- DOI: 10.1126/sciadv.ade3559
TREM2 inhibition triggers antitumor cell activity of myeloid cells in glioblastoma
Erratum in
-
Erratum for the Research Article: "TREM2 inhibition triggers antitumor cell activity of myeloid cells in glioblastoma" by Sun et al.Sci Adv. 2024 May 24;10(21):eadq2160. doi: 10.1126/sciadv.adq2160. Epub 2024 May 24. Sci Adv. 2024. PMID: 38787960 Free PMC article. No abstract available.
Abstract
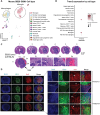
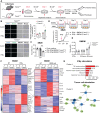
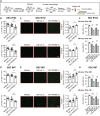
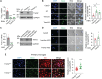
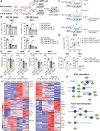
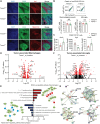
Triggering receptor expressed on myeloid cells 2 (TREM2) plays important roles in brain microglial function in neurodegenerative diseases, but the role of TREM2 in the GBM TME has not been examined. Here, we found that TREM2 is highly expressed in myeloid subsets, including macrophages and microglia in human and mouse GBM tumors and that high TREM2 expression correlates with poor prognosis in patients with GBM. TREM2 loss of function in human macrophages and mouse myeloid cells increased interferon-γ-induced immunoactivation, proinflammatory polarization, and tumoricidal capacity. In orthotopic mouse GBM models, mice with chronic and acute Trem2 loss of function exhibited decreased tumor growth and increased survival. Trem2 inhibition reprogrammed myeloid phenotypes and increased programmed cell death protein 1 (PD-1)+CD8+ T cells in the TME. Last, Trem2 deficiency enhanced the effectiveness of anti-PD-1 treatment, which may represent a therapeutic strategy for patients with GBM.
Figures




References
-
- Stupp R., Hegi M. E., Mason W. P., van den Bent M. J., Taphoorn M. J., Janzer R. C., Ludwin S. K., Allgeier A., Fisher B., Belanger K., Hau P., Brandes A. A., Gijtenbeek J., Marosi C., Vecht C. J., Mokhtari K., Wesseling P., Villa S., Eisenhauer E., Gorlia T., Weller M., Lacombe D., Cairncross J. G., Mirimanoff R. O.; on behalf of the European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; the National Cancer Institute of Canada Clinical Trials Group , Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10, 459–466 (2009). - PubMed
-
- Friebel E., Kapolou K., Unger S., Nunez N. G., Utz S., Rushing E. J., Regli L., Weller M., Greter M., Tugues S., Neidert M. C., Becher B., Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell 181, 1626–1642.e20 (2020). - PubMed
-
- Klemm F., Maas R. R., Bowman R. L., Kornete M., Soukup K., Nassiri S., Brouland J. P., Iacobuzio-Donahue C. A., Brennan C., Tabar V., Gutin P. H., Daniel R. T., Hegi M. E., Joyce J. A., Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell 181, 1643–1660.e17 (2020). - PMC - PubMed
-
- Ulland T. K., Song W. M., Huang S. C., Ulrich J. D., Sergushichev A., Beatty W. L., Loboda A. A., Zhou Y., Cairns N. J., Kambal A., Loginicheva E., Gilfillan S., Cella M., Virgin H. W., Unanue E. R., Wang Y., Artyomov M. N., Holtzman D. M., Colonna M., TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 170, 649–663.e13 (2017). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

