An in vivo neuroimmune organoid model to study human microglia phenotypes
- PMID: 37172564
- PMCID: PMC10284271
- DOI: 10.1016/j.cell.2023.04.022
An in vivo neuroimmune organoid model to study human microglia phenotypes
Abstract
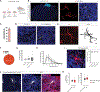
Microglia are specialized brain-resident macrophages that play crucial roles in brain development, homeostasis, and disease. However, until now, the ability to model interactions between the human brain environment and microglia has been severely limited. To overcome these limitations, we developed an in vivo xenotransplantation approach that allows us to study functionally mature human microglia (hMGs) that operate within a physiologically relevant, vascularized immunocompetent human brain organoid (iHBO) model. Our data show that organoid-resident hMGs gain human-specific transcriptomic signatures that closely resemble their in vivo counterparts. In vivo two-photon imaging reveals that hMGs actively engage in surveilling the human brain environment, react to local injuries, and respond to systemic inflammatory cues. Finally, we demonstrate that the transplanted iHBOs developed here offer the unprecedented opportunity to study functional human microglia phenotypes in health and disease and provide experimental evidence for a brain-environment-induced immune response in a patient-specific model of autism with macrocephaly.
Keywords: Human microglia; autism spectrum disorders; brain organoids; iPSCs; microglia in vivo identity; microglia surveillance; neuro-immune interactions; organoid transplantation; xenotransplantation.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


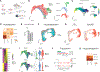


Comment in
-
Context matters: hPSC-derived microglia thrive in a humanized brain environment in vivo.Cell Stem Cell. 2023 Jul 6;30(7):909-910. doi: 10.1016/j.stem.2023.05.013. Cell Stem Cell. 2023. PMID: 37419102 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

