CFAP45, a heterotaxy and congenital heart disease gene, affects cilia stability
- PMID: 37172641
- PMCID: PMC10373286
- DOI: 10.1016/j.ydbio.2023.04.006
CFAP45, a heterotaxy and congenital heart disease gene, affects cilia stability
Abstract
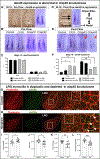
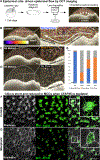
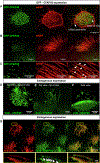
Congenital heart disease (CHD) is the most common and lethal birth defect, affecting 1.3 million individuals worldwide. During early embryogenesis, errors in Left-Right (LR) patterning called Heterotaxy (Htx) can lead to severe CHD. Many of the genetic underpinnings of Htx/CHD remain unknown. In analyzing a family with Htx/CHD using whole-exome sequencing, we identified a homozygous recessive missense mutation in CFAP45 in two affected siblings. CFAP45 belongs to the coiled-coil domain-containing protein family, and its role in development is emerging. When we depleted Cfap45 in frog embryos, we detected abnormalities in cardiac looping and global markers of LR patterning, recapitulating the patient's heterotaxy phenotype. In vertebrates, laterality is broken at the Left-Right Organizer (LRO) by motile monocilia that generate leftward fluid flow. When we analyzed the LRO in embryos depleted of Cfap45, we discovered "bulges" within the cilia of these monociliated cells. In addition, epidermal multiciliated cells lost cilia with Cfap45 depletion. Via live confocal imaging, we found that Cfap45 localizes in a punctate but static position within the ciliary axoneme, and depletion leads to loss of cilia stability and eventual detachment from the cell's apical surface. This work demonstrates that in Xenopus, Cfap45 is required to sustain cilia stability in multiciliated and monociliated cells, providing a plausible mechanism for its role in heterotaxy and congenital heart disease.
Keywords: CFAP45; Cilia; Congenital heart disease; Heterotaxy; Xenopus.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest SAL and MKK are co-founders of Victory Genomics, Inc.
Figures




Similar articles
-
CACNA1G, A Heterotaxy Candidate Gene, Plays a Role in Ciliogenesis and Left-Right Patterning in Xenopus tropicalis.Genesis. 2025 Feb;63(1):e70009. doi: 10.1002/dvg.70009. Genesis. 2025. PMID: 40008628
-
The heterotaxy gene GALNT11 glycosylates Notch to orchestrate cilia type and laterality.Nature. 2013 Dec 19;504(7480):456-9. doi: 10.1038/nature12723. Epub 2013 Nov 13. Nature. 2013. PMID: 24226769 Free PMC article.
-
WDR5 regulates left-right patterning via chromatin-dependent and -independent functions.Development. 2018 Nov 28;145(23):dev159889. doi: 10.1242/dev.159889. Development. 2018. PMID: 30377171 Free PMC article.
-
Left-right patterning in congenital heart disease beyond heterotaxy.Am J Med Genet C Semin Med Genet. 2020 Mar;184(1):90-96. doi: 10.1002/ajmg.c.31768. Epub 2020 Jan 30. Am J Med Genet C Semin Med Genet. 2020. PMID: 31999049 Free PMC article. Review.
-
The genetic landscape of cardiovascular left-right patterning defects.Curr Opin Genet Dev. 2022 Aug;75:101937. doi: 10.1016/j.gde.2022.101937. Epub 2022 Jun 28. Curr Opin Genet Dev. 2022. PMID: 35777348 Free PMC article. Review.
Cited by
-
Modelling human genetic disorders in Xenopus tropicalis.Dis Model Mech. 2024 May 1;17(5):dmm050754. doi: 10.1242/dmm.050754. Epub 2024 Jun 4. Dis Model Mech. 2024. PMID: 38832520 Free PMC article. Review.
-
Identification of the principal neuropeptide MIP and its action pathway in larval settlement of the echiuran worm Urechis unicinctus.BMC Genomics. 2024 Apr 3;25(1):337. doi: 10.1186/s12864-024-10228-y. BMC Genomics. 2024. PMID: 38641568 Free PMC article.
-
A new perspective on selenium's impact on renal function: European population-based analysis of plasma proteome-mediated Mendelian randomization study.Front Endocrinol (Lausanne). 2024 Sep 12;15:1410463. doi: 10.3389/fendo.2024.1410463. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39329105 Free PMC article.
-
CC2D1A causes ciliopathy, intellectual disability, heterotaxy, renal dysplasia, and abnormal CSF flow.Life Sci Alliance. 2024 Aug 21;7(10):e202402708. doi: 10.26508/lsa.202402708. Print 2024 Oct. Life Sci Alliance. 2024. PMID: 39168639 Free PMC article.
-
Study on Potential Differentially Expressed Genes in Idiopathic Pulmonary Fibrosis by Bioinformatics and Next-Generation Sequencing Data Analysis.Biomedicines. 2023 Nov 21;11(12):3109. doi: 10.3390/biomedicines11123109. Biomedicines. 2023. PMID: 38137330 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

