Comparison of the 2022 and 2017 European LeukemiaNet risk classifications in a real-life cohort of the PETHEMA group
- PMID: 37173322
- PMCID: PMC10182047
- DOI: 10.1038/s41408-023-00835-5
Comparison of the 2022 and 2017 European LeukemiaNet risk classifications in a real-life cohort of the PETHEMA group
Abstract
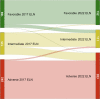
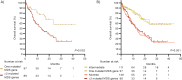
Next-Generation Sequencing is needed for the accurate genetic risk stratification of acute myeloid leukemia according to European LeukemiaNet (ELN) guidelines. We validated and compared the 2022 ELN risk classification in a real-life cohort of 546 intensively and 379 non-intensively treated patients. Among fit patients, those aged ≥65 years old showed worse OS than younger regardless risk classification. Compared with the 2017 classification, 14.5% of fit patients changed the risk with the 2022 classification, increasing the high-risk group from 44.3% to 51.8%. 3.7% and 0.9% FLT3-ITD mutated patients were removed from the favorable and adverse 2017 categories respectively to 2022 intermediate risk group. We suggest that midostaurin therapy could be a predictor for 3 years OS (85.2% with vs. 54.8% without midostaurin, P = 0.04). Forty-seven (8.6%) patients from the 2017 intermediate group were assigned to the 2022 adverse-risk group as they harbored myelodysplasia (MDS)-related mutations. Patients with one MDS-related mutation did not reach median OS, while patients with ≥2 mutations had 13.6 months median OS (P = 0.002). Patients with TP53 ± complex karyotype or inv(3) had a dismal prognosis (7.1 months median OS). We validate the prognostic utility of the 2022 ELN classification in a real-life setting providing supportive evidences to improve risk stratification guidelines.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and Management of AML in Adults: 2022 ELN Recommendations from an International Expert Panel. Blood. 2022 [cited 2 August 2022]; Available at https://pubmed.ncbi.nlm.nih.gov/35797463/. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

