Obesity impairs cardiolipin-dependent mitophagy and therapeutic intercellular mitochondrial transfer ability of mesenchymal stem cells
- PMID: 37173333
- PMCID: PMC10181927
- DOI: 10.1038/s41419-023-05810-3
Obesity impairs cardiolipin-dependent mitophagy and therapeutic intercellular mitochondrial transfer ability of mesenchymal stem cells
Abstract
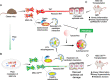
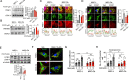
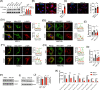
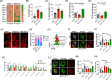
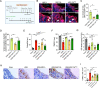
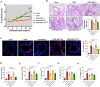
Mesenchymal stem cell (MSC) transplantation alleviates metabolic defects in diseased recipient cells by intercellular mitochondrial transport (IMT). However, the effect of host metabolic conditions on IMT and thereby on the therapeutic efficacy of MSCs has largely remained unexplored. Here we found impaired mitophagy, and reduced IMT in MSCs derived from high-fat diet (HFD)-induced obese mouse (MSC-Ob). MSC-Ob failed to sequester their damaged mitochondria into LC3-dependent autophagosomes due to decrease in mitochondrial cardiolipin content, which we propose as a putative mitophagy receptor for LC3 in MSCs. Functionally, MSC-Ob exhibited diminished potential to rescue mitochondrial dysfunction and cell death in stress-induced airway epithelial cells. Pharmacological modulation of MSCs enhanced cardiolipin-dependent mitophagy and restored their IMT ability to airway epithelial cells. Therapeutically, these modulated MSCs attenuated features of allergic airway inflammation (AAI) in two independent mouse models by restoring healthy IMT. However, unmodulated MSC-Ob failed to do so. Notably, in human (h)MSCs, induced metabolic stress associated impaired cardiolipin-dependent mitophagy was restored upon pharmacological modulation. In summary, we have provided the first comprehensive molecular understanding of impaired mitophagy in obese-derived MSCs and highlight the importance of pharmacological modulation of these cells for therapeutic intervention. A MSCs obtained from (HFD)-induced obese mice (MSC-Ob) show underlying mitochondrial dysfunction with a concomitant decrease in cardiolipin content. These changes prevent LC3-cardiolipin interaction, thereby reducing dysfunctional mitochondria sequestration into LC3-autophagosomes and thus impaired mitophagy. The impaired mitophagy is associated with reduced intercellular mitochondrial transport (IMT) via tunneling nanotubes (TNTs) between MSC-Ob and epithelial cells in co-culture or in vivo. B Pyrroloquinoline quinone (PQQ) modulation in MSC-Ob restores mitochondrial health, cardiolipin content, and thereby sequestration of depolarized mitochondria into the autophagosomes to alleviate impaired mitophagy. Concomitantly, MSC-Ob shows restoration of mitochondrial health upon PQQ treatment (MSC-ObPQQ). During co-culture with epithelial cells or transplantation in vivo into the mice lungs, MSC-ObPQQ restores IMT and prevents epithelial cell death. C Upon transplantation in two independent allergic airway inflammatory mouse models, MSC-Ob failed to rescue the airway inflammation, hyperactivity, metabolic changes in epithelial cells. D PQQ modulated MSCs restored these metabolic defects and restored lung physiology and airway remodeling parameters.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

