Immunosuppression for immune-related adverse events during checkpoint inhibition: an intricate balance
- PMID: 37173424
- PMCID: PMC10182067
- DOI: 10.1038/s41698-023-00380-1
Immunosuppression for immune-related adverse events during checkpoint inhibition: an intricate balance
Abstract
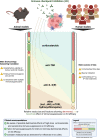
Immune checkpoint inhibitors (ICIs) have changed perspectives for patients with cancer, but come with severe immune-related adverse events (irAEs). To prevent fatality or chronicity, these irAEs are often promptly treated with high-dose immunosuppressants. Until recently, evidence on the effects of irAE management on ICI efficacy has been sparse. As a result, algorithms for irAE management are mostly expert-opinion based and barely consider possible detrimental effects of immunosuppressants on ICI efficacy. However, recent growing evidence suggests that vigorous immunosuppressive management of irAEs comes with unfavourable effects on ICI efficacy and survival. With expansion of the indications of ICIs, evidence-based treatment of irAEs without hampering tumour control becomes more and more important. In this review, we discuss novel evidence from pre-clinical and clinical studies on the effects of different irAE management regimens including corticosteroids, TNF inhibition and tocilizumab on cancer control and survival. We provide recommendations for pre-clinical research, cohort studies and clinical trials that can help clinicians in tailored irAE management, minimising patients' burden while maintaining ICI efficacy.
© 2023. The Author(s).
Conflict of interest statement
K.P.M.S. has advisory relationships with Bristol Myers Squibb, Novartis, MSD, Pierre Fabre and AbbVie and received honoraria from Novartis, MSD and Roche. Research funding: BMS, Philips and TigaTx are All paid to the institution. F.v.W. has received advisory/speaker fees from Takeda and Johnson&Johnson, and has received research funding from BMS, Takeda, Sanofi, Pfizer, Galapagos and Leo Pharma. No potential conflicts of interest were disclosed by the other authors.
Figures



References
-
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. U.S. Department of Health and Human Services (2017).
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

