Brachyury-targeted immunotherapy combined with gemcitabine against head and neck cancer
- PMID: 37173455
- PMCID: PMC10991094
- DOI: 10.1007/s00262-023-03460-0
Brachyury-targeted immunotherapy combined with gemcitabine against head and neck cancer
Abstract
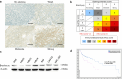
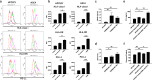
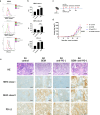
Brachyury is a transcription factor belonging to the T-box gene family and is involved in the posterior formation of the mesoderm and differentiation of chordates. As the overexpression of Brachyury is a poor prognostic factor in a variety of cancers, the establishment of Brachyury-targeted therapy would be beneficial for the treatment of aggressive tumors. Because transcription factors are difficult to treat with a therapeutic antibody, peptide vaccines are a feasible approach for targeting Brachyury. In this study, we identified Brachyury-derived epitopes that elicit antigen-specific and tumor-reactive CD4+ T cells that directly kill tumors. T cells recognizing Brachyury epitopes were present in patients with head and neck squamous cell carcinoma. Next, we focused on gemcitabine (GEM) as an immunoadjuvant to augment the efficacy of antitumor responses by T cells. Interestingly, GEM upregulated HLA class I and HLA-DR expression in tumor, followed by the upregulation of anti-tumor T cell responses. As tumoral PD-L1 expression was also augmented by GEM, PD-1/PD-L1 blockade and GEM synergistically enhanced the tumor-reactivity of Brachyury-reactive T cells. The synergy between the PD-1/PD-L1 blockade and GEM was also confirmed in a mouse model of head and neck squamous cell carcinoma. These results suggest that the combined treatment of Brachyury peptide with GEM and immune checkpoint blockade could be a promising immunotherapy against head and neck cancer.
Keywords: Brachyury; Gemcitabine; Head and neck squamous cell carcinoma; Immunotherapy; Peptide vaccine; Tumor-associated antigen.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

