Implementing an exclusive human milk diet for preterm infants: real-world experience in diverse NICUs
- PMID: 37173652
- PMCID: PMC10176849
- DOI: 10.1186/s12887-023-04047-5
Implementing an exclusive human milk diet for preterm infants: real-world experience in diverse NICUs
Abstract
Background: Human milk-based human milk fortifier (HMB-HMF) makes it possible to provide an exclusive human milk diet (EHMD) to very low birth weight (VLBW) infants in neonatal intensive care units (NICUs). Before the introduction of HMB-HMF in 2006, NICUs relied on bovine milk-based human milk fortifiers (BMB-HMFs) when mother's own milk (MOM) or pasteurized donor human milk (PDHM) could not provide adequate nutrition. Despite evidence supporting the clinical benefits of an EHMD (such as reducing the frequency of morbidities), barriers prevent its widespread adoption, including limited health economics and outcomes data, cost concerns, and lack of standardized feeding guidelines.
Methods: Nine experts from seven institutions gathered for a virtual roundtable discussion in October 2020 to discuss the benefits and challenges to implementing an EHMD program in the NICU environment. Each center provided a review of the process of starting their program and also presented data on various neonatal and financial metrics associated with the program. Data gathered were either from their own Vermont Oxford Network outcomes or an institutional clinical database. As each center utilizes their EHMD program in slightly different populations and over different time periods, data presented was center-specific. After all presentations, the experts discussed issues within the field of neonatology that need to be addressed with regards to the utilization of an EHMD in the NICU population.
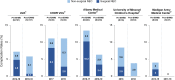
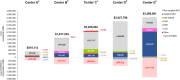
Results: Implementation of an EHMD program faces many barriers, no matter the NICU size, patient population or geographic location. Successful implementation requires a team approach (including finance and IT support) with a NICU champion. Having pre-specified target populations as well as data tracking is also helpful. Real-world experiences of NICUs with established EHMD programs show reductions in comorbidities, regardless of the institution's size or level of care. EHMD programs also proved to be cost effective. For the NICUs that had necrotizing enterocolitis (NEC) data available, EHMD programs resulted in either a decrease or change in total (medical + surgical) NEC rate and reductions in surgical NEC. Institutions that provided cost and complications data all reported a substantial cost avoidance after EHMD implementation, ranging between $515,113 and $3,369,515 annually per institution.
Conclusions: The data provided support the initiation of EHMD programs in NICUs for very preterm infants, but there are still methodologic issues to be addressed so that guidelines can be created and all NICUs, regardless of size, can provide standardized care that benefits VLBW infants.
Keywords: Advocacy; Cost; Exclusive human milk diet; Growth; Human milk fortifier; Implementation; NICU; Necrotizing enterocolitis; Preterm birth; Public policy.
© 2023. The Author(s).
Conflict of interest statement
Dr. Swanson has received speaker’s honoraria from Mednax, Inc. and Prolacta Bioscience. All other authors declare no competing interests.
Figures
References
-
- Agostini C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50:85–91. doi: 10.1097/MPG.0b013e3181adaee0. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

