High-Dosage NMN Promotes Ferroptosis to Suppress Lung Adenocarcinoma Growth through the NAM-Mediated SIRT1-AMPK-ACC Pathway
- PMID: 37173894
- PMCID: PMC10177531
- DOI: 10.3390/cancers15092427
High-Dosage NMN Promotes Ferroptosis to Suppress Lung Adenocarcinoma Growth through the NAM-Mediated SIRT1-AMPK-ACC Pathway
Abstract
Background: Nicotinamide mononucleotide (NMN) is the physiological circulating NAD precursor thought to elevate the cellular level of NAD+ and to ameliorate various age-related diseases. An inseparable link exists between aging and tumorigenesis, especially involving aberrant energetic metabolism and cell fate regulation in cancer cells. However, few studies have directly investigated the effects of NMN on another major ageing-related disease: tumors.
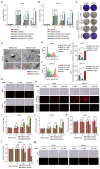
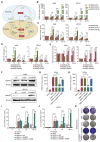
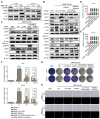
Methods: We conducted a series of cell and mouse models to evaluate the anti-tumor effect of high-dose NMN. Transmission electron microscopy and a Mito-FerroGreen-labeled immunofluorescence assay (Fe2+) were utilized to demonstrate ferroptosis. The metabolites of NAM were detected via ELISA. The expression of the proteins involved in the SIRT1-AMPK-ACC signaling were detected using a Western blot assay.
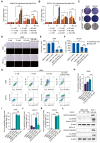
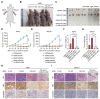
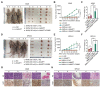
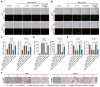
Results: The results showed that high-dose NMN inhibits lung adenocarcinoma growth in vitro and in vivo. Excess NAM is produced through the metabolism of high-dose NMN, whereas the overexpression of NAMPT significantly decreases intracellular NAM content, which, in turn, boosts cell proliferation. Mechanistically, high-dose NMN promotes ferroptosis through NAM-mediated SIRT1-AMPK-ACC signaling.
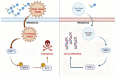
Conclusions: This study highlights the tumor influence of NMN at high doses in the manipulation of cancer cell metabolism, providing a new perspective on clinical therapy in patients with lung adenocarcinoma.
Keywords: NAM; NAMPT; NMN; ferroptosis; lung adenocarcinoma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

