Integrins and the Metastasis-like Dissemination of Acute Lymphoblastic Leukemia to the Central Nervous System
- PMID: 37173970
- PMCID: PMC10177281
- DOI: 10.3390/cancers15092504
Integrins and the Metastasis-like Dissemination of Acute Lymphoblastic Leukemia to the Central Nervous System
Abstract
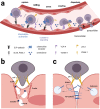
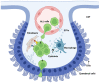
Acute lymphoblastic leukemia (ALL) disseminates with high prevalence to the central nervous system (CNS) in a process resembling aspects of the CNS surveillance of normal immune cells as well as aspects of brain metastasis from solid cancers. Importantly, inside the CNS, the ALL blasts are typically confined within the cerebrospinal fluid (CSF)-filled cavities of the subarachnoid space, which they use as a sanctuary protected from both chemotherapy and immune cells. At present, high cumulative doses of intrathecal chemotherapy are administered to patients, but this is associated with neurotoxicity and CNS relapse still occurs. Thus, it is imperative to identify markers and novel therapy targets specific to CNS ALL. Integrins represent a family of adhesion molecules involved in cell-cell and cell-matrix interactions, implicated in the adhesion and migration of metastatic cancer cells, normal immune cells, and leukemic blasts. The ability of integrins to also facilitate cell-adhesion mediated drug resistance, combined with recent discoveries of integrin-dependent routes of leukemic cells into the CNS, have sparked a renewed interest in integrins as markers and therapeutic targets in CNS leukemia. Here, we review the roles of integrins in CNS surveillance by normal lymphocytes, dissemination to the CNS by ALL cells, and brain metastasis from solid cancers. Furthermore, we discuss whether ALL dissemination to the CNS abides by known hallmarks of metastasis, and the potential roles of integrins in this context.
Keywords: CNS; acute lymphoblastic leukemia; immune surveillance; integrin; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Toft N., Birgens H., Abrahamsson J., Griškevičius L., Hallböök H., Heyman M., Klausen T.W., Jónsson Ó.G., Palk K., Pruunsild K., et al. Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia. Leukemia. 2018;32:606–615. doi: 10.1038/leu.2017.265. - DOI - PubMed
-
- Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

