Thyroid Hormone Withdrawal versus Recombinant Human TSH as Preparation for I-131 Therapy in Patients with Metastatic Thyroid Cancer: A Systematic Review and Meta-Analysis
- PMID: 37173976
- PMCID: PMC10177224
- DOI: 10.3390/cancers15092510
Thyroid Hormone Withdrawal versus Recombinant Human TSH as Preparation for I-131 Therapy in Patients with Metastatic Thyroid Cancer: A Systematic Review and Meta-Analysis
Abstract
Background: Differentiated thyroid carcinoma (DTC) is characterized by an excellent prognosis with a 10-year survival rate > 90%. However, when DTC develops in a metastatic form, it has been shown to significantly impact patient survival and quality of life. Although I-131 has been shown to be an effective therapy in patients with metastatic DTC, whether its efficacy after recombinant human TSH (rhTSH) is comparable to endogenous TSH stimulation by thyroid hormone deprivation (THW) is still debated. Our present study was prompted to compare clinical results obtained in metastatic DTC by I-131 administered after rhTSH and THW stimulation protocols, respectively.
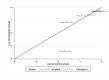
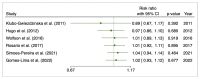
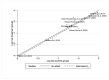
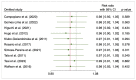
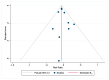
Methods: A systematic search on PubMed, Web of Science, and Scopus was performed from January to February 2023. Pooled risk ratios with 95% CI were determined for evaluating the initial response after to I-131 therapy after preparation with rhTSH or THW and the disease progression. To track the accumulation of evidence and reduce type I errors because of small data, a cumulative meta-analysis was performed. A sensitivity analysis was also performed to examine the impact of individual studies on overall prevalence results.
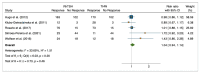
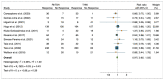
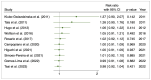
Results: Ten studies were included with a total of 1929 patients pre-treated with rhTSH (n = 953) and THW (n = 976), respectively. The cumulative data of our systematic review and meta-analysis showed an increase in the risk ratio over the years without any change in favour of a pre-treatment or the other on the effectiveness of I-131 therapy of metastatic DTC.
Conclusions: Our data suggest that pretreatment with rhTSH or THW has no significant impact on the effectiveness of I-131 therapy for metastatic DTC. This implies that concerns about the use of one or the other pretreatment should be deferred to clinical evaluations made considering patient characteristics and reduction in side effects.
Keywords: 131I therapy; differentiated thyroid carcinoma; recombinant human-thyroid stimulating hormone; thyroid hormone withdrawal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Surveillance, Epidemiology, and End Results Program. Thyroid Cancer—Cancer Stat Facts. [(accessed on 23 March 2023)]; Available online: https://seer.cancer.gov/statfacts/html/thyro.html.
-
- Carhill A.A., Litofsky D.R., Ross D.S., Jonklaas J., Cooper D.S., Brierley J.D., Ladenson P.W., Ain K.B., Fein H.G., Haugen B.R., et al. Long-Term Outcomes Following Therapy in Differentiated Thyroid Carcinoma: NTCTCS Registry Analysis 1987-2012. J. Clin. Endocrinol. Metab. 2015;100:3270–3279. doi: 10.1210/JC.2015-1346. - DOI - PMC - PubMed
-
- Miller J.E., Al-Attar N.C., Brown O.H., Shaughness G.G., Rosculet N.P., Avram A.M., Hughes D.T. Location and Causation of Residual Lymph Node Metastasis After Surgical Treatment of Regionally Advanced Differentiated Thyroid Cancer. Thyroid. 2018;28:593–600. doi: 10.1089/thy.2017.0434. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

