Comments on and Illustrations of the EFSUMB CEUS Guidelines: Transabdominal and Endoscopic Ultrasound Features of Intrapancreatic Metastases and the Role of Multiparametric Imaging and EUS-Guided Sampling in Rare Pancreatic Tumors
- PMID: 37174015
- PMCID: PMC10177255
- DOI: 10.3390/cancers15092546
Comments on and Illustrations of the EFSUMB CEUS Guidelines: Transabdominal and Endoscopic Ultrasound Features of Intrapancreatic Metastases and the Role of Multiparametric Imaging and EUS-Guided Sampling in Rare Pancreatic Tumors
Abstract
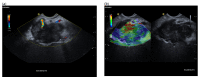
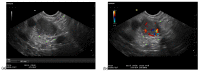
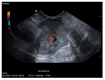
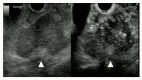
A definite pathologic diagnosis of intrapancreatic metastasis is crucial for the management decision, i.e., curative or palliative surgery versus chemotherapy or conservative/palliative therapy. This review focuses on the appearance of intrapancreatic metastases on native and contrast-enhanced transabdominal ultrasound and endoscopic ultrasound. Differences and similarities in relation to the primary tumor, and the differential diagnosis from pancreatic carcinoma and neuroendocrine neoplasms are described. The frequency of intrapancreatic metastases in autopsy studies and surgical resection studies will be discussed. Further emphasis is placed on endoscopic ultrasound-guided sampling to confirm the diagnosis.
Keywords: CEUS; CH-EUS; EUS-guided sampling; intrapancreatic metastases; prevalence.
Conflict of interest statement
The authors declare no conflict of interest.
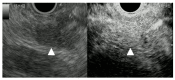
Figures


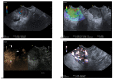
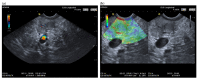

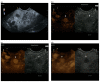
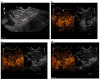
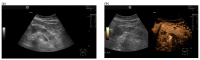

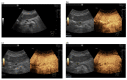

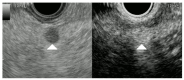
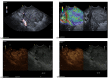


Similar articles
-
Comments and illustrations of the European Federation of Societies for Ultrasound in Medicine contrast-enhanced ultrasound guidelines. Rare pancreatic tumors, imaging features on transabdominal ultrasound and EUS with contrast enhancement: Rare epithelial pancreatic tumors: solid pseudopapillary neoplasm, acinar cell carcinoma, mixed neuroendocrine-non-neuroendocrine neoplasms, some rare subtypes of pancreatic adenocarcinoma and pancreatoblastoma.Endosc Ultrasound. 2024 May-Jun;13(3):129-144. doi: 10.1097/eus.0000000000000056. Epub 2024 Apr 30. Endosc Ultrasound. 2024. PMID: 39318646 Free PMC article.
-
Comments and illustrations of the European Federation of Societies for Ultrasound in Medicine contrast-enhanced ultrasonography guidelines: multiparametric imaging and EUS-guided sampling in rare pancreatic tumors. Mesenchymal pancreatic tumors of intermediate biological behaviour.Endosc Ultrasound. 2024 May-Jun;13(3):145-153. doi: 10.1097/eus.0000000000000071. Epub 2024 Jul 5. Endosc Ultrasound. 2024. PMID: 39318650 Free PMC article.
-
Comments and illustrations of the European Federation of Societies for Ultrasound in Medicine contrast-enhanced ultrasound guidelines: Multiparametric imaging and EUS-guided sampling in rare pancreatic tumors. Benign mesenchymal pancreatic tumors.Endosc Ultrasound. 2024 Jul-Aug;13(4):218-231. doi: 10.1097/eus.0000000000000070. Epub 2024 Aug 16. Endosc Ultrasound. 2024. PMID: 39318747 Free PMC article.
-
Comments and Illustrations of the European Federation of Societies for Ultrasound in Medicine (EFSUMB) Guidelines: Rare Malignant Pulmonal and Pleural Tumors: Primary Pulmonary Sarcoma and Mesothelioma, Imaging Features on Transthoracic Ultrasound.Diagnostics (Basel). 2024 Oct 21;14(20):2339. doi: 10.3390/diagnostics14202339. Diagnostics (Basel). 2024. PMID: 39451662 Free PMC article. Review.
-
Contrast-enhanced endoscopic ultrasound.Endosc Ultrasound. 2012 Oct;1(3):130-6. doi: 10.7178/eus.03.003. Endosc Ultrasound. 2012. PMID: 24949350 Free PMC article. Review.
Cited by
-
Pancreatic ultrasound: An update of measurements, reference values, and variations of the pancreas.Ultrasound Int Open. 2024 Oct 14;10:a23899085. doi: 10.1055/a-2389-9085. eCollection 2024. Ultrasound Int Open. 2024. PMID: 39411753 Free PMC article. Review.
-
The diagnostic value of endoscopic ultrasound for esophageal subepithelial lesions: A review.Medicine (Baltimore). 2024 Nov 15;103(46):e40419. doi: 10.1097/MD.0000000000040419. Medicine (Baltimore). 2024. PMID: 39560558 Free PMC article. Review.
-
Comments and illustrations of the European Federation of Societies for Ultrasound in Medicine contrast-enhanced ultrasound guidelines. Rare pancreatic tumors, imaging features on transabdominal ultrasound and EUS with contrast enhancement: Rare epithelial pancreatic tumors: solid pseudopapillary neoplasm, acinar cell carcinoma, mixed neuroendocrine-non-neuroendocrine neoplasms, some rare subtypes of pancreatic adenocarcinoma and pancreatoblastoma.Endosc Ultrasound. 2024 May-Jun;13(3):129-144. doi: 10.1097/eus.0000000000000056. Epub 2024 Apr 30. Endosc Ultrasound. 2024. PMID: 39318646 Free PMC article.
-
Comments and illustrations of the European Federation of Societies for Ultrasound in Medicine contrast-enhanced ultrasonography guidelines: multiparametric imaging and EUS-guided sampling in rare pancreatic tumors. Mesenchymal pancreatic tumors of intermediate biological behaviour.Endosc Ultrasound. 2024 May-Jun;13(3):145-153. doi: 10.1097/eus.0000000000000071. Epub 2024 Jul 5. Endosc Ultrasound. 2024. PMID: 39318650 Free PMC article.
References
-
- Claudon M., Dietrich C.F., Choi B.I., Cosgrove D.O., Kudo M., Nolsoe C.P., Piscaglia F., Wilson S.R., Barr R.G., Chammas M.C., et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver-update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med. Biol. 2013;39:187–210. doi: 10.1016/j.ultrasmedbio.2012.09.002. - DOI - PubMed
-
- Dietrich C.F., Nolsoe C.P., Barr R.G., Berzigotti A., Burns P.N., Cantisani V., Chammas M.C., Chaubal N., Choi B.I., Clevert D.A., et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020;46:2579–2604. doi: 10.1016/j.ultrasmedbio.2020.04.030. - DOI - PubMed
-
- Piscaglia F., Nolsoe C., Dietrich C.F., Cosgrove D.O., Gilja O.H., Bachmann Nielsen M., Albrecht T., Barozzi L., Bertolotto M., Catalano O., et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. - DOI - PubMed
-
- Sidhu P.S., Cantisani V., Dietrich C.F., Gilja O.H., Saftoiu A., Bartels E., Bertolotto M., Calliada F., Clevert D.A., Cosgrove D., et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version) Ultraschall Med. 2018;39:e2–e44. doi: 10.1055/a-0586-1107. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

