Extracellular Vesicles Potentiate Medulloblastoma Metastasis in an EMMPRIN and MMP-2 Dependent Manner
- PMID: 37174066
- PMCID: PMC10177484
- DOI: 10.3390/cancers15092601
Extracellular Vesicles Potentiate Medulloblastoma Metastasis in an EMMPRIN and MMP-2 Dependent Manner
Abstract
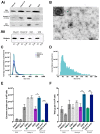
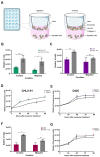
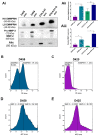
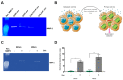
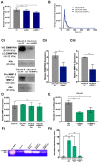
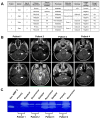
Extracellular vesicles (EVs) have emerged as pivotal mediators of communication in the tumour microenvironment. More specifically, nanosized extracellular vesicles termed exosomes have been shown to contribute to the establishment of a premetastatic niche. Here, we sought to determine what role exosomes play in medulloblastoma (MB) progression and elucidate the underlying mechanisms. Metastatic MB cells (D458 and CHLA-01R) were found to secrete markedly more exosomes compared to their nonmetastatic, primary counterparts (D425 and CHLA-01). In addition, metastatic cell-derived exosomes significantly enhanced the migration and invasiveness of primary MB cells in transwell migration assays. Protease microarray analysis identified that matrix metalloproteinase-2 (MMP-2) was enriched in metastatic cells, and zymography and flow cytometry assays of metastatic exosomes demonstrated higher levels of functionally active MMP-2 on their external surface. Stable genetic knockdown of MMP-2 or extracellular matrix metalloproteinase inducer (EMMPRIN) in metastatic MB cells resulted in the loss of this promigratory effect. Analysis of serial patient cerebrospinal fluid (CSF) samples showed an increase in MMP-2 activity in three out of four patients as the tumour progressed. This study demonstrates the importance of EMMPRIN and MMP-2-associated exosomes in creating a favourable environment to drive medulloblastoma metastasis via extracellular matrix signalling.
Keywords: EMMPRIN; MMP-2; exosomes; extracellular vesicles; medulloblastoma; metastasis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Hill R.M., Richardson S., Schwalbe E.C., Hicks D., Lindsey J.C., Crosier S., Rafiee G., Grabovska Y., Wharton S.B., Jacques T.S., et al. Time, Pattern, and Outcome of Medulloblastoma Relapse and Their Association with Tumour Biology at Diagnosis and Therapy: A Multicentre Cohort Study. Lancet Child Adolesc. Health. 2020;4:865–874. doi: 10.1016/S2352-4642(20)30246-7. - DOI - PMC - PubMed
-
- Ramaswamy V., Remke M., Bouffet E., Bailey S., Clifford S.C., Doz F., Kool M., Dufour C., Vassal G., Milde T., et al. Risk Stratification of Childhood Medulloblastoma in the Molecular Era: The Current Consensus. Acta Neuropathol. 2016;131:821–831. doi: 10.1007/s00401-016-1569-6. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

