Endorsement of TNBC Biomarkers in Precision Therapy by Nanotechnology
- PMID: 37174125
- PMCID: PMC10177107
- DOI: 10.3390/cancers15092661
Endorsement of TNBC Biomarkers in Precision Therapy by Nanotechnology
Abstract
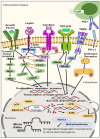
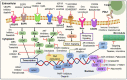
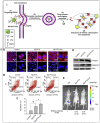
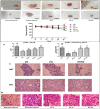
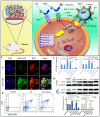
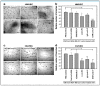
Breast cancer is a heterogeneous disease which accounts globally for approximately 1 million new cases annually, wherein more than 200,000 of these cases turn out to be cases of triple-negative breast cancer (TNBC). TNBC is an aggressive and rare breast cancer subtype that accounts for 10-15% of all breast cancer cases. Chemotherapy remains the only therapy regimen against TNBC. However, the emergence of innate or acquired chemoresistance has hindered the chemotherapy used to treat TNBC. The data obtained from molecular technologies have recognized TNBC with various gene profiling and mutation settings that have helped establish and develop targeted therapies. New therapeutic strategies based on the targeted delivery of therapeutics have relied on the application of biomarkers derived from the molecular profiling of TNBC patients. Several biomarkers have been found that are targets for the precision therapy in TNBC, such as EGFR, VGFR, TP53, interleukins, insulin-like growth factor binding proteins, c-MET, androgen receptor, BRCA1, glucocorticoid, PTEN, ALDH1, etc. This review discusses the various candidate biomarkers identified in the treatment of TNBC along with the evidence supporting their use. It was established that nanoparticles had been considered a multifunctional system for delivering therapeutics to target sites with increased precision. Here, we also discuss the role of biomarkers in nanotechnology translation in TNBC therapy and management.
Keywords: biomarkers; nanoparticles; personalized therapy; targeted therapy; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Shohdy K.S., Almeldin D.S., Fekry M.A., Ismail M.A., AboElmaaref N.A., ElSadany E.G., Hamza B.M., El-Shorbagy F.H., Ali A.S., Attia H., et al. Pathological responses and survival outcomes in patients with locally advanced breast cancer after neoadjuvant chemotherapy: A single-institute experience. J. Egypt. Natl. Cancer Inst. 2021;33:39. doi: 10.1186/s43046-021-00096-y. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

