Development and Application of a Physiologically Based Pharmacokinetic Model for Diclazuril in Broiler Chickens
- PMID: 37174549
- PMCID: PMC10177140
- DOI: 10.3390/ani13091512
Development and Application of a Physiologically Based Pharmacokinetic Model for Diclazuril in Broiler Chickens
Abstract
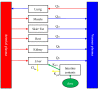
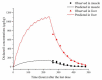
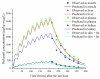
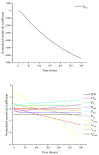
Withdrawal periods for diclazuril in broilers have traditionally been determined through regression analysis. However, over the last two decades, the physiologically based pharmacokinetic (PBPK) model has gained prominence as a predictive tool for veterinary drug residues, which offers an alternative method for establishing appropriate withdrawal periods for veterinary drugs. In this current study, a flow-limited PBPK model was developed to predict diclazuril concentrations in broilers following long-duration administration via medicated feed and water. This model consists of nine compartments, including arterial and venous plasma, lung, muscle, skin + fat, kidney, liver, intestine contents, and the rest of the body compartment. Physiological parameters such as tissue weights (Vcxx) and blood flow (Qcxx) were gathered from published studies, and tissue/plasma partition coefficients (Pxx) were calculated through the area method or parameter optimization. Published diclazuril concentrations were compared to the predicted values, indicating the accuracy and validity of the model. The sensitivity analysis showed that parameters associated with cardiac output, drug absorption, and elimination significantly affected diclazuril concentrations in the muscle. Finally, a Monte Carlo analysis, consisting of 1000 iterations, was conducted to calculate the withdrawal period. Based on the Chinese MRL values, we calculated a withdrawal period of 0 days for both recommended dosing regimens (through mediated water and feed at concentrations of 0.5-1 mg/L and 1 mg/kg, respectively). However, based on the European MRLs, longer periods were determined for the mediated feed dosing route. Our model provides a foundation for scaling other coccidiostats and poultry species.
Keywords: broiler chickens; diclazuril; physiologically based pharmacokinetic (PBPK) model; residue prediction; withdrawal interval.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- CCVP (Commission of Chinese Veterinary Pharmacopoeia) The People’s Republic of China Veterinary Pharmacopoeia. 2020th ed. Volume 1. China Agriculture Press; Beijing, China: 2021. pp. 94–96.
-
- WHO (World Health Organization) Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Diclazuril (WHO Food Additives Series 41) [(accessed on 20 March 2023)]. Available online: https://inchem.org/documents/jecfa/jecmono/v041je08.htm.
Grants and funding
LinkOut - more resources
Full Text Sources

