Glycyrrhizic Acid Prevents Paclitaxel-Induced Neuropathy via Inhibition of OATP-Mediated Neuronal Uptake
- PMID: 37174648
- PMCID: PMC10177491
- DOI: 10.3390/cells12091249
Glycyrrhizic Acid Prevents Paclitaxel-Induced Neuropathy via Inhibition of OATP-Mediated Neuronal Uptake
Abstract
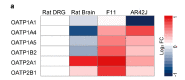
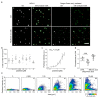
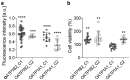
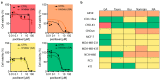
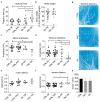
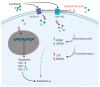
Peripheral neuropathy is a common side effect of cancer treatment with paclitaxel. The mechanisms by which paclitaxel is transported into neurons, which are essential for preventing neuropathy, are not well understood. We studied the uptake mechanisms of paclitaxel into neurons using inhibitors for endocytosis, autophagy, organic anion-transporting polypeptide (OATP) drug transporters, and derivatives of paclitaxel. RT-qPCR was used to investigate the expression levels of OATPs in different neuronal tissues and cell lines. OATP transporters were pharmacologically inhibited or modulated by overexpression and CRISPR/Cas9-knock-out to investigate paclitaxel transport in neurons. Through these experiments, we identified OATP1A1 and OATP1B2 as the primary neuronal transporters for paclitaxel. In vitro inhibition of OATP1A1 and OAT1B2 by glycyrrhizic acid attenuated neurotoxicity, while paclitaxel's antineoplastic effects were sustained in cancer cell lines. In vivo, glycyrrhizic acid prevented paclitaxel-induced toxicity and improved behavioral and electrophysiological measures. This study indicates that a set of OATPs are involved in paclitaxel transport into neurons. The inhibition of OATP1A1 and OATP1B2 holds a promising strategy to prevent paclitaxel-induced peripheral neuropathy.
Keywords: CIPN; OATP; OATP1A1; OATP1B2; drug transport; glycyrrhizic acid; paclitaxel; taxol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


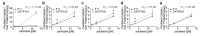

Similar articles
-
Expression of organic anion-transporting polypeptides 1B1 and 1B3 in ovarian cancer cells: relevance for paclitaxel transport.Biomed Pharmacother. 2011 Sep;65(6):417-26. doi: 10.1016/j.biopha.2011.04.031. Epub 2011 Jun 12. Biomed Pharmacother. 2011. PMID: 21719246
-
Organic anion-transporting polypeptides contribute to the hepatic uptake of berberine.Xenobiotica. 2015;45(12):1138-46. doi: 10.3109/00498254.2015.1042537. Epub 2015 Jun 11. Xenobiotica. 2015. PMID: 26068524
-
OATP1B2 deficiency protects against paclitaxel-induced neurotoxicity.J Clin Invest. 2018 Feb 1;128(2):816-825. doi: 10.1172/JCI96160. Epub 2018 Jan 16. J Clin Invest. 2018. PMID: 29337310 Free PMC article.
-
Role for Drug Transporters in Chemotherapy-Induced Peripheral Neuropathy.Clin Transl Sci. 2021 Mar;14(2):460-467. doi: 10.1111/cts.12915. Epub 2020 Nov 9. Clin Transl Sci. 2021. PMID: 33142018 Free PMC article. Review.
-
Organic Anion Transporting Polypeptide (OATP) transporter expression, localization and function in the human intestine.Pharmacol Ther. 2019 Mar;195:39-53. doi: 10.1016/j.pharmthera.2018.10.007. Epub 2018 Oct 19. Pharmacol Ther. 2019. PMID: 30347208 Review.
Cited by
-
Overcoming Resistance in Prostate Cancer Therapy Using a DZ-Simvastatin Conjugate.Mol Pharm. 2024 Feb 5;21(2):873-882. doi: 10.1021/acs.molpharmaceut.3c00993. Epub 2024 Jan 16. Mol Pharm. 2024. PMID: 38229228 Free PMC article.
-
Unveiling the mechanisms and challenges of cancer drug resistance.Cell Commun Signal. 2024 Feb 12;22(1):109. doi: 10.1186/s12964-023-01302-1. Cell Commun Signal. 2024. PMID: 38347575 Free PMC article. Review.
-
Chemotherapy-Induced Peripheral Neuropathy: A Recent Update on Pathophysiology and Treatment.Life (Basel). 2024 Aug 9;14(8):991. doi: 10.3390/life14080991. Life (Basel). 2024. PMID: 39202733 Free PMC article. Review.
-
Crossing the Blood-Brain Barrier: Innovations in Receptor- and Transporter-Mediated Transcytosis Strategies.Pharmaceutics. 2025 May 28;17(6):706. doi: 10.3390/pharmaceutics17060706. Pharmaceutics. 2025. PMID: 40574019 Free PMC article. Review.
-
Advances in the roles of glycyrrhizic acid in cancer therapy.Front Pharmacol. 2023 Aug 15;14:1265172. doi: 10.3389/fphar.2023.1265172. eCollection 2023. Front Pharmacol. 2023. PMID: 37649893 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

