Zbtb40 Deficiency Leads to Morphological and Phenotypic Abnormalities of Spermatocytes and Spermatozoa and Causes Male Infertility
- PMID: 37174664
- PMCID: PMC10177581
- DOI: 10.3390/cells12091264
Zbtb40 Deficiency Leads to Morphological and Phenotypic Abnormalities of Spermatocytes and Spermatozoa and Causes Male Infertility
Abstract
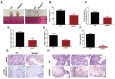
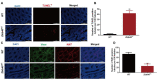
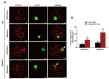
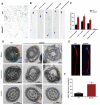
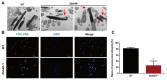
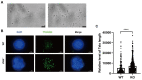
Studies on the gene regulation of spermatogenesis are of unusual significance for maintaining male reproduction and treating male infertility. Here, we have demonstrated, for the first time, that a loss of ZBTB40 function leads to abnormalities in the morphological and phenotypic characteristics of mouse spermatocytes and spermatids as well as male infertility. We revealed that Zbtb40 was expressed in spermatocytes of mouse testes, and it was co-localized with γH2AX in mouse secondary spermatocytes. Interestingly, spermatocytes of Zbtb40 knockout mice had longer telomeres, compromised double-strand break (DSB) repair in the sex chromosome, and a higher apoptosis ratio compared to wild-type (WT) mice. The testis weight, testicular volume, and cauda epididymis body weight of the Zbtb40+/- male mice were significantly lower than in WT mice. Mating tests indicated that Zbtb40+/- male mice were able to mate normally, but they failed to produce any pups. Notably, sperm of Zbtb40+/- mice showed flagellum deformities and abnormal acrosome biogenesis. Furthermore, a ZBTB40 mutation was associated with non-obstructive azoospermia. Our results implicate that ZBTB40 deficiency leads to morphological and phenotypic abnormalities of spermatocytes and spermatids and causes male infertility. This study thus offers a new genetic mechanism regulating mammalian spermatogenesis and provides a novel target for gene therapy in male infertility.
Keywords: ZBTB40 mutation; Zbtb40 knockout; male infertility; spermatids; spermatocytes; telomere length.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Seipin deficiency increases chromocenter fragmentation and disrupts acrosome formation leading to male infertility.Cell Death Dis. 2015 Jul 16;6(7):e1817. doi: 10.1038/cddis.2015.188. Cell Death Dis. 2015. PMID: 26181198 Free PMC article.
-
Abnormal spermatogenesis and male infertility in testicular zinc finger protein Zfp318-knockout mice.Dev Growth Differ. 2016 Sep;58(7):600-8. doi: 10.1111/dgd.12301. Epub 2016 Jul 7. Dev Growth Differ. 2016. PMID: 27385512 Free PMC article.
-
Systematic characterization of human testis-specific actin capping protein β3 as a possible biomarker for male infertility.Hum Reprod. 2017 Mar 1;32(3):514-522. doi: 10.1093/humrep/dew353. Hum Reprod. 2017. PMID: 28104696
-
Molecular genetics of infertility: loss-of-function mutations in humans and corresponding knockout/mutated mice.Hum Reprod Update. 2021 Jan 4;27(1):154-189. doi: 10.1093/humupd/dmaa034. Hum Reprod Update. 2021. PMID: 33118031 Review.
-
Genetic mutations resulting in estrogen insufficiency in the male.Mol Cell Endocrinol. 1998 Oct 25;145(1-2):55-9. doi: 10.1016/s0303-7207(98)00169-5. Mol Cell Endocrinol. 1998. PMID: 9922099 Review.
Cited by
-
Zinc Finger and BTB Domain-Containing 20: A Newly Emerging Player in Pathogenesis and Development of Human Cancers.Biomolecules. 2024 Feb 4;14(2):192. doi: 10.3390/biom14020192. Biomolecules. 2024. PMID: 38397429 Free PMC article. Review.
-
Extensive binding of uncharacterized human transcription factors to genomic dark matter.bioRxiv [Preprint]. 2024 Nov 12:2024.11.11.622123. doi: 10.1101/2024.11.11.622123. bioRxiv. 2024. PMID: 39605320 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

