Cellular Mechanisms Mediating Exercise-Induced Protection against Cardiotoxic Anthracycline Cancer Therapy
- PMID: 37174712
- PMCID: PMC10177216
- DOI: 10.3390/cells12091312
Cellular Mechanisms Mediating Exercise-Induced Protection against Cardiotoxic Anthracycline Cancer Therapy
Abstract
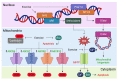
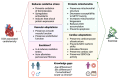
Anthracyclines such as doxorubicin are widely used chemotherapy drugs. A common side effect of anthracycline therapy is cardiotoxicity, which can compromise heart function and lead to dilated cardiomyopathy and heart failure. Dexrazoxane and heart failure medications (i.e., beta blockers and drugs targeting the renin-angiotensin system) are prescribed for the primary prevention of cancer therapy-related cardiotoxicity and for the management of cardiac dysfunction and symptoms if they arise during chemotherapy. However, there is a clear need for new therapies to combat the cardiotoxic effects of cancer drugs. Exercise is a cardioprotective stimulus that has recently been shown to improve heart function and prevent functional disability in breast cancer patients undergoing anthracycline chemotherapy. Evidence from preclinical studies supports the use of exercise training to prevent or attenuate the damaging effects of anthracyclines on the cardiovascular system. In this review, we summarise findings from experimental models which provide insight into cellular mechanisms by which exercise may protect the heart from anthracycline-mediated damage, and identify knowledge gaps that require further investigation. Improved understanding of the mechanisms by which exercise protects the heart from anthracyclines may lead to the development of novel therapies to treat cancer therapy-related cardiotoxicity.
Keywords: cardioprotection; cardiotoxicity; exercise; exerkines; sex differences.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Larsen C.M., Arango M.G., Dasari H., Calle M.A., Adjei E., Mesa J.R., Scott C.G., Thompson C.A., Cerhan J.R., Haddad T.C., et al. Association of Anthracycline with Heart Failure in Patients Treated for Breast Cancer or Lymphoma, 1985–2010. JAMA Netw. Open. 2023;6:e2254669. doi: 10.1001/jamanetworkopen.2022.54669. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

