Poly-L-Lactic Acid Fillers Improved Dermal Collagen Synthesis by Modulating M2 Macrophage Polarization in Aged Animal Skin
- PMID: 37174720
- PMCID: PMC10177436
- DOI: 10.3390/cells12091320
Poly-L-Lactic Acid Fillers Improved Dermal Collagen Synthesis by Modulating M2 Macrophage Polarization in Aged Animal Skin
Abstract
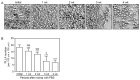
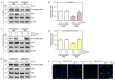
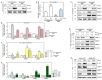
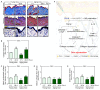
Poly-L-lactic acid (PLLA) fillers correct cutaneous volume loss by stimulating fibroblasts to synthesize collagen and by augmenting the volume. PLLA triggers the macrophage-induced activation of fibroblasts that secrete transforming growth factor-β (TGF-β). However, whether M2 macrophage polarization is involved in PLLA-induced collagen synthesis via fibroblast activation in aged skin is not known. Therefore, we evaluated the effect of PLLA on dermal collagen synthesis via M2 polarization in an H2O2-induced cellular senescence model and aged animal skin. H2O2-treated macrophages had increased expression levels of the M1 marker CD80 and decreased expression levels of the M2 marker CD163, which were reversed by PLLA. The expression levels of interleukin (IL)-4 and IL-13, which mediate M2 polarization, were decreased in H2O2-treated macrophages and increased upon the PLLA treatment. CD163, IL-4, and IL-13 expression levels were decreased in aged skin, but increased after the PLLA treatment. The expression levels of TGF-β, pSMAD2/SMAD2, connective tissue growth factor (CTGF), alpha-smooth muscle actin (α-SMA), collagen type 1A1 (COL1A1), and COL3A1 were also decreased in aged skin, but increased after the PLLA treatment. Moreover, PLLA upregulated phosphatidylinositol 3-kinase p85α (PI3-kinase p85α)/protein kinase B (AKT) signaling, leading to fibroblast proliferation. PLLA decreased the expression of matrix metalloproteinase (MMP) 2 and MMP3, which destroy collagen and elastin fibers in aged skin. The amount of collagen and elastin fibers in aged skin increased following the PLLA treatment. In conclusion, PLLA causes M2 polarization by increasing IL-4 and IL-13 levels and upregulating TGF-β expression and collagen synthesis in aged skin.
Keywords: M2 polarization; collagen and elastin synthesis; poly-L-lactic acid; senescence.
Conflict of interest statement
Kyunghee Byun has received research grants from SACCI Bio Co.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

