The Pesticide Chlordecone Promotes Parkinsonism-like Neurodegeneration with Tau Lesions in Midbrain Cultures and C. elegans Worms
- PMID: 37174736
- PMCID: PMC10177284
- DOI: 10.3390/cells12091336
The Pesticide Chlordecone Promotes Parkinsonism-like Neurodegeneration with Tau Lesions in Midbrain Cultures and C. elegans Worms
Abstract
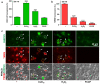
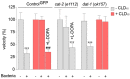
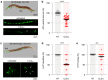
Chlordecone (CLD) is an organochlorine pesticide (OCP) that is currently banned but still contaminates ecosystems in the French Caribbean. Because OCPs are known to increase the risk of Parkinson's disease (PD), we tested whether chronic low-level intoxication with CLD could reproduce certain key characteristics of Parkinsonism-like neurodegeneration. For that, we used culture systems of mouse midbrain dopamine (DA) neurons and glial cells, together with the nematode C. elegans as an in vivo model organism. We established that CLD kills cultured DA neurons in a concentration- and time-dependent manner while exerting no direct proinflammatory effects on glial cells. DA cell loss was not impacted by the degree of maturation of the culture. The use of fluorogenic probes revealed that CLD neurotoxicity was the consequence of oxidative stress-mediated insults and mitochondrial disturbances. In C. elegans worms, CLD exposure caused a progressive loss of DA neurons associated with locomotor deficits secondary to alterations in food perception. L-DOPA, a molecule used for PD treatment, corrected these deficits. Cholinergic and serotoninergic neuronal cells were also affected by CLD in C. elegans, although to a lesser extent than DA neurons. Noticeably, CLD also promoted the phosphorylation of the aggregation-prone protein tau (but not of α-synuclein) both in midbrain cell cultures and in a transgenic C. elegans strain expressing a human form of tau in neurons. In summary, our data suggest that CLD is more likely to promote atypical forms of Parkinsonism characterized by tau pathology than classical synucleinopathy-associated PD.
Keywords: Parkinsonism; cell culture model; chlordecone; dopamine neurons; neurodegeneration; tauopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Dereumeaux C., Saoudi A., Guldner L., Pecheux M., Chesneau J., Thomé J.-P., Ledrans M., Tertre A.L., Denys S., Fillol C. Chlordecone and Organochlorine Compound Levels in the French West Indies Population in 2013–2014. Environ. Sci. Pollut. Res. Int. 2020;27:41033–41045. doi: 10.1007/s11356-019-07181-9. - DOI - PubMed
-
- Boucher O., Simard M.-N., Muckle G., Rouget F., Kadhel P., Bataille H., Chajès V., Dallaire R., Monfort C., Thomé J.-P., et al. Exposure to an Organochlorine Pesticide (Chlordecone) and Development of 18-Month-Old Infants. Neurotoxicology. 2013;35:162–168. doi: 10.1016/j.neuro.2013.01.007. - DOI - PubMed
-
- Dallaire R., Muckle G., Rouget F., Kadhel P., Bataille H., Guldner L., Seurin S., Chajès V., Monfort C., Boucher O., et al. Cognitive, Visual, and Motor Development of 7-Month-Old Guadeloupean Infants Exposed to Chlordecone. Environ. Res. 2012;118:79–85. doi: 10.1016/j.envres.2012.07.006. - DOI - PubMed
-
- Taylor J.R. Neurological Manifestations in Humans Exposed to Chlordecone and Follow-up Results. Neurotoxicology. 1982;3:9–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

