A Turn-On Quinazolinone-Based Fluorescence Probe for Selective Detection of Carbon Monoxide
- PMID: 37175064
- PMCID: PMC10180483
- DOI: 10.3390/molecules28093654
A Turn-On Quinazolinone-Based Fluorescence Probe for Selective Detection of Carbon Monoxide
Abstract
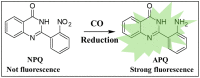
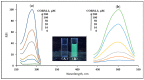
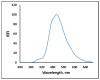
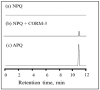
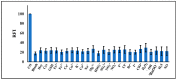
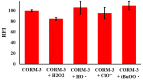
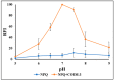
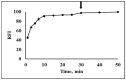
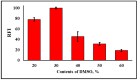
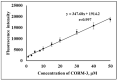
Carbon monoxide (CO) is a toxic, hazardous gas that has a colorless and odorless nature. On the other hand, CO possesses some physiological roles as a signaling molecule that regulates neurotransmitters in addition to its hazardous effects. Because of the dual nature of CO, there is a need to develop a sensitive, selective, and rapid method for its detection. Herein, we designed and synthesized a turn-on fluorescence probe, 2-(2'-nitrophenyl)-4(3H)-quinazolinone (NPQ), for the detection of CO. NPQ provided a turn-on fluorescence response to CO and the fluorescence intensity at 500 nm was increased with increasing the concentration of CO. This fluorescence enhancement could be attributed to the conversion of the nitro group of NPQ to an amino group by the reducing ability of CO. The fluorescence assay for CO using NPQ as a reagent was confirmed to have a good linear relationship in the range of 1.0 to 50 µM with an excellent correlation coefficient (r) of 0.997 and good sensitivity down to a limit of detection at 0.73 µM (20 ppb) defined as mean blank+3SD. Finally, we successfully applied NPQ to the preparation of a test paper that can detect CO generated from charcoal combustion.
Keywords: carbon monoxide; fluorescence probe; metal free; quinazolinone; test paper.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Palladacycle Based Fluorescence Turn-On Probe for Sensitive Detection of Carbon Monoxide.ACS Sens. 2018 Feb 23;3(2):285-289. doi: 10.1021/acssensors.7b00835. Epub 2018 Feb 6. ACS Sens. 2018. PMID: 29392928
-
A metal-free coumarin-based fluorescent probe for the turn-on monitoring of carbon monoxide in an aqueous solution and living cells.Analyst. 2021 Feb 22;146(4):1289-1294. doi: 10.1039/d0an02107e. Analyst. 2021. PMID: 33350408
-
Developed a novel quinazolinone based turn-on fluorescence probe for highly selective monitoring hypochlorite and its bioimaging applications.Spectrochim Acta A Mol Biomol Spectrosc. 2020 Mar 5;228:117845. doi: 10.1016/j.saa.2019.117845. Epub 2019 Nov 22. Spectrochim Acta A Mol Biomol Spectrosc. 2020. PMID: 31784226
-
A novel red emission fluorescent probe for monitoring carbon monoxide in living cells and zebrafish.Anal Methods. 2021 Jul 7;13(25):2871-2877. doi: 10.1039/d1ay00704a. Epub 2021 Jun 7. Anal Methods. 2021. PMID: 34096941
-
Carbon Monoxide Poisoning.Crit Care Clin. 2021 Jul;37(3):657-672. doi: 10.1016/j.ccc.2021.03.010. Crit Care Clin. 2021. PMID: 34053712 Review.
Cited by
-
A Tale of Two Cities in Fluorescent Sensing of Carbon Monoxide: Probes That Detect CO and Those That Detect Only Chemically Reactive CO Donors (CORMs), but Not CO.J Org Chem. 2024 Dec 20;89(24):17891-17909. doi: 10.1021/acs.joc.4c02301. Epub 2024 Nov 14. J Org Chem. 2024. PMID: 39540647 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

