A Novel Paclitaxel Derivative for Triple-Negative Breast Cancer Chemotherapy
- PMID: 37175072
- PMCID: PMC10180349
- DOI: 10.3390/molecules28093662
A Novel Paclitaxel Derivative for Triple-Negative Breast Cancer Chemotherapy
Abstract
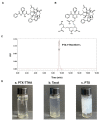
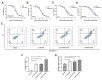
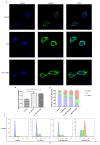
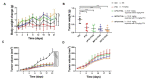
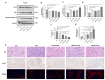
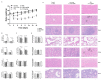
Paclitaxel-triethylenetetramine hexaacetic acid conjugate (PTX-TTHA), a novel semi-synthetic taxane, is designed to improve the water solubility and cosolvent toxicity of paclitaxel in several aminopolycarboxylic acid groups. In this study, the in vitro and in vivo antitumor effects and mechanisms of PTX-TTHA against triple-negative breast cancer (TNBC) and its intravenous toxicity were evaluated. Results showed the water solubility of PTX-TTHA was greater than 5 mg/mL, which was about 7140-fold higher than that of paclitaxel (<0.7 µg/mL). PTX-TTHA (10-105 nmol/L) could significantly inhibit breast cancer proliferation and induce apoptosis by stabilizing microtubules and arresting the cell cycle in the G2/M phase in vitro, with its therapeutic effect and mechanism similar to paclitaxel. However, when the MDA-MB-231 cell-derived xenograft (CDX) tumor model received PTX-TTHA (13.73 mg/kg) treatment once every 3 days for 21 days, the tumor inhibition rate was up to 77.32%. Furthermore, PTX-TTHA could inhibit tumor proliferation by downregulating Ki-67, and induce apoptosis by increasing pro-apoptotic proteins (Bax, cleaved caspase-3) and TdT-mediated dUTP nick end labeling (TUNEL) positive apoptotic cells, and reducing anti-apoptotic protein (Bcl-2). Moreover, PTX-TTHA demonstrated no sign of acute toxicity on vital organs, hematological, and biochemical parameters at the limit dose (138.6 mg/kg, i.v.). Our study indicated that PTX-TTHA showed better water solubility than paclitaxel, as well as comparable in vitro and in vivo antitumor activity in TNBC models. In addition, the antitumor mechanism of PTX-TTHA was related to microtubule regulation and apoptosis signaling pathway activation.
Keywords: antitumor; apoptosis; microtubule stabilization; proliferation; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Targeting EGFR of triple-negative breast cancer enhances the therapeutic efficacy of paclitaxel- and cetuximab-conjugated nanodiamond nanocomposite.Acta Biomater. 2019 Mar 1;86:395-405. doi: 10.1016/j.actbio.2019.01.025. Epub 2019 Jan 16. Acta Biomater. 2019. PMID: 30660004
-
RAD6 inhibition enhances paclitaxel sensitivity of triple negative breast cancer cells by aggravating mitotic spindle damage.BMC Cancer. 2022 Oct 18;22(1):1073. doi: 10.1186/s12885-022-10119-z. BMC Cancer. 2022. PMID: 36258187 Free PMC article.
-
Bcl-2 Up-Regulation Mediates Taxane Resistance Downstream of APC Loss.Int J Mol Sci. 2024 Jun 19;25(12):6745. doi: 10.3390/ijms25126745. Int J Mol Sci. 2024. PMID: 38928449 Free PMC article.
-
Discovery of a transforming growth factor-β1 inhibitory peptide, Charis 1000 to enhance the therapeutic efficacy of paclitaxel in triple-negative breast cancer.Int J Biol Macromol. 2025 Jun;314:144216. doi: 10.1016/j.ijbiomac.2025.144216. Epub 2025 May 14. Int J Biol Macromol. 2025. PMID: 40379179
-
Research progress of paclitaxel nanodrug delivery system in the treatment of triple-negative breast cancer.Mater Today Bio. 2024 Nov 23;29:101358. doi: 10.1016/j.mtbio.2024.101358. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39677523 Free PMC article. Review.
Cited by
-
Paclitaxel and its semi-synthetic derivatives: comprehensive insights into chemical structure, mechanisms of action, and anticancer properties.Eur J Med Res. 2024 Jan 30;29(1):90. doi: 10.1186/s40001-024-01657-2. Eur J Med Res. 2024. PMID: 38291541 Free PMC article. Review.
-
Shape Matters: Impact of Mesoporous Silica Nanoparticle Morphology on Anti-Tumor Efficacy.Pharmaceutics. 2024 May 8;16(5):632. doi: 10.3390/pharmaceutics16050632. Pharmaceutics. 2024. PMID: 38794294 Free PMC article.
-
Advances in nanomaterials for precision drug delivery: Insights into pharmacokinetics and toxicity.Bioimpacts. 2024 Nov 2;15:30573. doi: 10.34172/bi.30573. eCollection 2025. Bioimpacts. 2024. PMID: 40256227 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

