PROTACs in the Management of Prostate Cancer
- PMID: 37175108
- PMCID: PMC10179857
- DOI: 10.3390/molecules28093698
PROTACs in the Management of Prostate Cancer
Abstract
Cancer treatments with targeted therapy have gained immense interest due to their low levels of toxicity and high selectivity. Proteolysis-Targeting Chimeras (PROTACs) have drawn special attention in the development of cancer therapeutics owing to their unique mechanism of action, their ability to target undruggable proteins, and their focused target engagement. PROTACs selectively degrade the target protein through the ubiquitin-proteasome system, which describes a different mode of action compared to conventional small-molecule inhibitors or even antibodies. Among different cancer types, prostate cancer (PC) is the most prevalent non-cutaneous cancer in men. Genetic alterations and the overexpression of several genes, such as FOXA1, AR, PTEN, RB1, TP53, etc., suppress the immune response, resulting in drug resistance to conventional drugs in prostate cancer. Since the progression of ARV-110 (PROTAC for PC) into clinical phases, the focus of research has quickly shifted to protein degraders targeting prostate cancer. The present review highlights an overview of PROTACs in prostate cancer and their superiority over conventional inhibitors. We also delve into the underlying pathophysiology of the disease and explain the structural design and linkerology strategies for PROTAC molecules. Additionally, we touch on the various targets for PROTAC in prostate cancer, including the androgen receptor (AR) and other critical oncoproteins, and discuss the future prospects and challenges in this field.
Keywords: PROTAC; androgen receptor; mutation; prostate cancer; resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




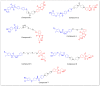

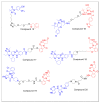
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

