Recent Progress in Photoresponsive Biomaterials
- PMID: 37175122
- PMCID: PMC10180172
- DOI: 10.3390/molecules28093712
Recent Progress in Photoresponsive Biomaterials
Abstract
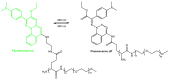
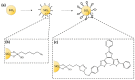
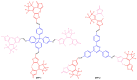
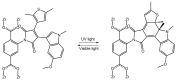
Photoresponsive biomaterials have garnered increasing attention recently due to their ability to dynamically regulate biological interactions and cellular behaviors in response to light. This review provides an overview of recent advances in the design, synthesis, and applications of photoresponsive biomaterials, including photochromic molecules, photocleavable linkers, and photoreactive polymers. We highlight the various approaches used to control the photoresponsive behavior of these materials, including modulation of light intensity, wavelength, and duration. Additionally, we discuss the applications of photoresponsive biomaterials in various fields, including drug delivery, tissue engineering, biosensing, and optical storage. A selection of significant cutting-edge articles collected in recent years has been discussed based on the structural pattern and light-responsive performance, focusing mainly on the photoactivity of azobenzene, hydrazone, diarylethenes, and spiropyrans, and the design of smart materials as the most targeted and desirable application. Overall, this review highlights the potential of photoresponsive biomaterials to enable spatiotemporal control of biological processes and opens up exciting opportunities for developing advanced biomaterials with enhanced functionality.
Keywords: azobenzene; biomaterials; diarylethene; hydrazones; photoresponsive; spiropyran.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


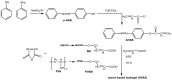
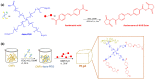
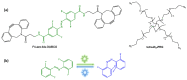
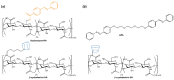















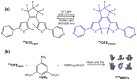

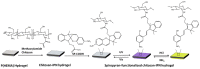

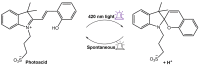

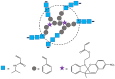

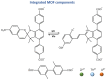
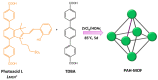
References
-
- Chang V.Y., Fedele C., Priimagi A., Shishido A., Barrett C.J. Photoreversible soft azo dye materials: Toward optical control of bio-interfaces. Adv. Opt. Mater. 2019;7:1900091. doi: 10.1002/adom.201900091. - DOI
-
- Keyvan Rad J., Balzade Z., Mahdavian A.R. Spiropyran-based advanced photoswitchable materials: A fascinating pathway to the future stimuli-responsive devices. J. Photochem. Photobiol. C Photochem. Rev. 2022;51:100487. doi: 10.1016/j.jphotochemrev.2022.100487. - DOI
-
- Di Martino M., Marrafino F., Diana R., Iannelli P., Concilio S. Advances in Bionanomaterials II: Selected Papers, Proceedings of the 3rd International Conference on Bio and Nanomaterials, BIONAM 2019, Mediterranean Sea, Italy, 29 September–3 October 2019. Springer; Berlin/Heidelberg, Germany: 2020. Fluorescent Probes for Applications in Bioimaging; pp. 243–258.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

