Thieno-Thiazolostilbenes, Thienobenzo-Thiazoles, and Naphtho-Oxazoles: Computational Study and Cholinesterase Inhibitory Activity
- PMID: 37175190
- PMCID: PMC10180155
- DOI: 10.3390/molecules28093781
Thieno-Thiazolostilbenes, Thienobenzo-Thiazoles, and Naphtho-Oxazoles: Computational Study and Cholinesterase Inhibitory Activity
Abstract
Naphtho-triazoles and thienobenzo-triazoles have so far proven to be very potent inhibitors of the enzyme butyrylcholinesterase (BChE). Based on these results, in this work, new thienobenzo-thiazoles were designed and synthesized, and their potential inhibitory activity was tested and compared with their analogs, naphtho-oxazoles. The synthesis was carried out by photochemical cyclization of thieno-thiazolostilbenes obtained in the first reaction step. Several thienobenzo-thiazoles and naphtho-oxazoles have shown significant potential as BChE inhibitors, together with the phenolic thiazolostilbene being the most active of all tested compounds. These results are significant as BChE has been attracting growing attention due to its positive role in the treatment of Alzheimer's disease. Computational examination based on the DFT approach enabled the characterization of the geometry and electronic structure of the studied molecules. Furthermore, the molecular docking study, accompanied by additional optimization of complexes ligand-active site, offered insight into the structure and stabilizing interactions in the complexes of studied molecules and BChE.
Keywords: 1,3-oxazole; 1,3-thiazole; cholinesterase inhibition; electronic structure; molecular docking; thiophene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






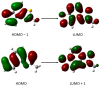

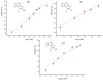




References
-
- Parekh N.M., Juddhawala K.V., Rawal B.M. Antimicrobial activity of thiazolyl benzenesulfonamide-condensed 2,4-thiazolidinediones derivatives. Med. Chem. Res. 2013;22:2737–2745. doi: 10.1007/s00044-012-0273-x. - DOI
-
- Rostom S.A., El-Ashmawy I.M., AbdElRazik H.A., Badr M.H., Ashour H.M. Design and synthesis of some thiazolyl and thiadiazolyl derivatives of antipyrine as potential non-acidic anti-inflammatory, analgesic and antimicrobial agents. Bioorg. Med. Chem. 2009;17:882–895. doi: 10.1016/j.bmc.2008.11.035. - DOI - PubMed
-
- Zablotskaya A., Segal I., Germane S., Shestakova I., Domracheva I., Nesterova A., Geronikaki A., Lukevics E. Silyl modification of biologically active compounds. Trimethylsilyl ethers of hydroxyl-containing thiazole derivatives. Chem. Heterocycl. Compd. 2002;38:859–866. doi: 10.1023/A:1020698107686. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

