Anticonvulsant Profile of Selected Medium-Chain Fatty Acids (MCFAs) Co-Administered with Metformin in Mice in Acute and Chronic Treatment
- PMID: 37175220
- PMCID: PMC10179922
- DOI: 10.3390/molecules28093810
Anticonvulsant Profile of Selected Medium-Chain Fatty Acids (MCFAs) Co-Administered with Metformin in Mice in Acute and Chronic Treatment
Abstract
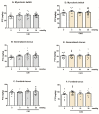
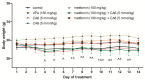
In contrast to the other components of the medium-chain triglycerides ketogenic diet (MCT KD), i.e., caprylic acid (CA8), a comprehensive evaluation of caproic (CA6) and lauric acids' (CA12) properties in standard chemical and electrical seizure tests in mice has not yet been performed. We investigated their effects in maximal electroshock seizure threshold (MEST), 6 Hz seizure threshold and intravenous (i.v.) pentylenetetrazole (PTZ) seizure tests. Since ketone body production can be regulated by the activation of 5'AMP-activated protein kinase (AMPK), we hypothesized that metformin (an AMPK activator) enhance ketogenesis and would act synergistically with the fatty acids to inhibit convulsions. We assessed the effects of acute and chronic co-treatment with metformin and CA6/CA8 on seizures. CA6 and CA12 (p.o.) increased seizure threshold in the 6 Hz seizure test. CA6 at the highest tested dose (30 mmol/kg) developed toxicity in several mice, impaired motor performance and induced ketoacidosis. Acute and chronic co-treatment with metformin and CA6/CA8 did not affect seizure thresholds. Moreover, we observed the pro-convulsive effect of the acute co-administration of CA8 (5 mmol/kg) and metformin (100 mg/kg). Since this co-treatment was pro-convulsive, the safety profile and risk/benefit ratio of MCT KD and metformin concomitant therapy in epileptic patients should be further evaluated.
Keywords: 6 Hz seizure test; MEST; caproic acid; caprylic acid; lauric acid; metformin; mice; pentylenetetrazole; seizure threshold; seizures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

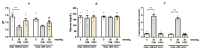




Similar articles
-
Acute anticonvulsant effects of capric acid in seizure tests in mice.Prog Neuropsychopharmacol Biol Psychiatry. 2015 Mar 3;57:110-6. doi: 10.1016/j.pnpbp.2014.10.013. Epub 2014 Oct 30. Prog Neuropsychopharmacol Biol Psychiatry. 2015. PMID: 25445478
-
Anticonvulsant profile of caprylic acid, a main constituent of the medium-chain triglyceride (MCT) ketogenic diet, in mice.Neuropharmacology. 2012 Mar;62(4):1882-9. doi: 10.1016/j.neuropharm.2011.12.015. Epub 2011 Dec 17. Neuropharmacology. 2012. PMID: 22210332
-
Evaluation of the Anticonvulsant Effect of Brilliant Blue G, a Selective P2X7 Receptor Antagonist, in the iv PTZ-, Maximal Electroshock-, and 6 Hz-Induced Seizure Tests in Mice.Neurochem Res. 2017 Nov;42(11):3114-3124. doi: 10.1007/s11064-017-2348-z. Epub 2017 Jul 12. Neurochem Res. 2017. PMID: 28702712 Free PMC article.
-
The Search for New Screening Models of Pharmacoresistant Epilepsy: Is Induction of Acute Seizures in Epileptic Rodents a Suitable Approach?Neurochem Res. 2017 Jul;42(7):1926-1938. doi: 10.1007/s11064-016-2025-7. Epub 2016 Aug 8. Neurochem Res. 2017. PMID: 27502939 Review.
-
Experimental Models for the Discovery of Novel Anticonvulsant Drugs: Focus on Pentylenetetrazole-Induced Seizures and Associated Memory Deficits.Curr Pharm Des. 2020;26(15):1693-1711. doi: 10.2174/1381612826666200131105324. Curr Pharm Des. 2020. PMID: 32003682 Review.
Cited by
-
In Silico Screening Identification of Fatty Acids and Fatty Acid Derivatives with Antiseizure Activity: In Vitro and In Vivo Validation.Pharmaceutics. 2024 Jul 27;16(8):996. doi: 10.3390/pharmaceutics16080996. Pharmaceutics. 2024. PMID: 39204342 Free PMC article.
References
-
- Wilder R.M. The effects of ketonemia on the course of epilepsy. Mayo Clin. Bull. 1921;2:307–308.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

