Synthesis and Structural Characterization of p-Carboranylamidine Derivatives
- PMID: 37175246
- PMCID: PMC10179778
- DOI: 10.3390/molecules28093837
Synthesis and Structural Characterization of p-Carboranylamidine Derivatives
Abstract
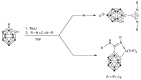
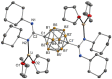
In this contribution, the first amidinate and amidine derivatives of p-carborane are described. Double lithiation of p-carborane (1) with n-butyllithium followed by treatment with 1,3-diorganocarbodiimides, R-N=C=N-R (R = iPr, Cy (= cyclohexyl)), in DME or THF afforded the new p-carboranylamidinate salts p-C2H10B10[C(NiPr)2Li(DME)]2 (2) and p-C2H10B10[C(NCy)2Li(THF)2]2 (3). Subsequent treatment of 2 and 3 with 2 equiv. of chlorotrimethylsilane (Me3SiCl) provided the silylated neutral bis(amidine) derivatives p-C2H10B10[C{iPrN(SiMe3)}(=NiPr)]2 (4) and p-C2H10B10[C{CyN(SiMe3)}(=NCy)]2 (5). The new compounds 3 and 4 have been structurally characterized by single-crystal X-ray diffraction. The lithium carboranylamidinate 3 comprises a rare trigonal planar coordination geometry around the lithium ions.
Keywords: boron; carborane; carboranylamidinate; lithiation; p-carborane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Synthesis and crystal structures of two new tin bis-(carboranylamidinate) complexes.Acta Crystallogr E Crystallogr Commun. 2017 Sep 12;73(Pt 10):1443-1448. doi: 10.1107/S2056989017012671. eCollection 2017 Oct 1. Acta Crystallogr E Crystallogr Commun. 2017. PMID: 29250355 Free PMC article.
-
Novel inorganic heterocycles from dimetalated carboranylamidinates.Dalton Trans. 2014 Apr 7;43(13):5001-13. doi: 10.1039/c3dt52751d. Dalton Trans. 2014. PMID: 24202239
-
Five different types of η(8)-cyclooctatetraenyl-lanthanide half-sandwich complexes from one ligand set, including a "giant neodymium wheel".Dalton Trans. 2016 Sep 14;45(34):13332-46. doi: 10.1039/c6dt01974a. Epub 2016 Jul 6. Dalton Trans. 2016. PMID: 27381428
-
Bulky Amidinate Complexes of Indium(III). Synthesis and Structure of [CyNC((t)Bu)NCy](2)InCl.Inorg Chem. 1996 Apr 24;35(9):2448-2451. doi: 10.1021/ic950943a. Inorg Chem. 1996. PMID: 11666455
-
Formation and structure of the first metal complexes comprising amidino-guanidinate ligands.Acta Crystallogr E Crystallogr Commun. 2016 Oct 4;72(Pt 11):1526-1531. doi: 10.1107/S2056989016015322. eCollection 2016 Nov 1. Acta Crystallogr E Crystallogr Commun. 2016. PMID: 27840700 Free PMC article.
Cited by
-
Structural Evolution of Small-Sized Phosphorus-Doped Boron Clusters: A Half-Sandwich-Structured PB15 Cluster.Molecules. 2024 Jul 18;29(14):3384. doi: 10.3390/molecules29143384. Molecules. 2024. PMID: 39064962 Free PMC article.
References
-
- Brown A.D., Colquhoun H.M., Daniels A.J., MacBride J.A.H., Stephenson I.R., Wade K. Polymers and ceramics based on icosahedral carboranes. Model studies of the formation and hydrolytic stability of aryl ether, ketone, amide and borane linkages between carborane units. J. Mater. Chem. 1992;2:793–804. doi: 10.1039/jm9920200793. - DOI
-
- Murphy D.M., Mingos D.M.P., Haggitt J.L., Poell H.R., Westcott S.A., Marder T.B., Taylor N.J., Kanis D.R. Synthesis of icosahedral carboranes for second-harmonic generation. Part 2. J. Mater. Chem. 1993;3:139–148. doi: 10.1039/jm9930300139. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

