Antibacterial and Antifungal Terpenes from the Medicinal Angiosperms of Asia and the Pacific: Haystacks and Gold Needles
- PMID: 37175283
- PMCID: PMC10180233
- DOI: 10.3390/molecules28093873
Antibacterial and Antifungal Terpenes from the Medicinal Angiosperms of Asia and the Pacific: Haystacks and Gold Needles
Abstract
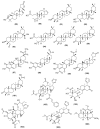
This review identifies terpenes isolated from the medicinal Angiosperms of Asia and the Pacific with antibacterial and/or antifungal activities and analyses their distribution, molecular mass, solubility, and modes of action. All data in this review were compiled from Google Scholar, PubMed, Science Direct, Web of Science, ChemSpider, PubChem, and library searches from 1968 to 2022. About 300 antibacterial and/or antifungal terpenes were identified during this period. Terpenes with a MIC ≤ 2 µg/mL are mostly amphiphilic and active against Gram-positive bacteria, with a molecular mass ranging from about 150 to 550 g/mol, and a polar surface area around 20 Ų. Carvacrol, celastrol, cuminol, dysoxyhainic acid I, ent-1β,14β-diacetoxy-7α-hydroxykaur-16-en-15-one, ergosterol-5,8-endoperoxide, geranylgeraniol, gossypol, 16α-hydroxy-cleroda-3,13 (14)Z-diene-15,16-olide, 7-hydroxycadalene, 17-hydroxyjolkinolide B, (20R)-3β-hydroxy-24,25,26,27-tetranor-5α cycloartan-23,21-olide, mansonone F, (+)-6,6'-methoxygossypol, polygodial, pristimerin, terpinen-4-ol, and α-terpineol are chemical frameworks that could be candidates for the further development of lead antibacterial or antifungal drugs.
Keywords: Asia; Pacific; antibacterial terpenes; antifungal terpenes; medicinal plants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- The Angiosperm Phylogeny Group. Chase M.W., Christenhusz M.J., Fay M.F., Byng J.W., Judd W.S., Soltis D.E., Mabberley D.J., Sennikov A.N., Soltis P.S., et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. 2016;181:1–20.
-
- Tiku A.R. In: Antimicrobial Compounds (Phytoanticipins and Phytoalexins) and Their Role in Plant Defense, in Co-Evolution of Secondary Metabolites. Mérillon J.-M., Ramawat K.G., editors. Springer International Publishing; Cham, Switzerland: 2020. pp. 845–868.
-
- Berg B.V.D. Bacterial cleanup: Lateral diffusion of hydrophobic molecules through protein channel walls. Biomol. 2010;1:263–270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

