Different In Silico Approaches Using Heterocyclic Derivatives against the Binding between Different Lineages of SARS-CoV-2 and ACE2
- PMID: 37175318
- PMCID: PMC10180195
- DOI: 10.3390/molecules28093908
Different In Silico Approaches Using Heterocyclic Derivatives against the Binding between Different Lineages of SARS-CoV-2 and ACE2
Abstract
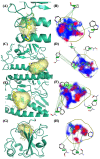
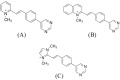
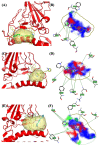
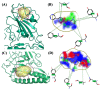
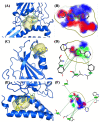
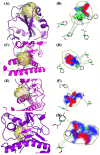
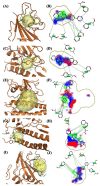
Over the last few years, the study of the SARS-CoV-2 spike protein and its mutations has become essential in understanding how it interacts with human host receptors. Since the crystallized structure of the spike protein bound to the angiotensin-converting enzyme 2 (ACE2) receptor was released (PDB code 6M0J), in silico studies have been performed to understand the interactions between these two proteins. Specifically, in this study, heterocyclic compounds with different chemical characteristics were examined to highlight the possibility of interaction with the spike protein and the disruption of the interaction between ACE2 and the spike protein. Our results showed that these compounds interacted with the spike protein and interposed in the interaction zone with ACE2. Although further studies are needed, this work points to these heterocyclic push-pull compounds as possible agents capable of interacting with the spike protein, with the potential for the inhibition of spike protein-ACE2 binding.
Keywords: SARS-CoV-2; heterocyclic derivatives; molecular modeling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

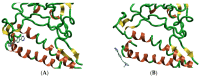


Similar articles
-
In silico investigation of critical binding pattern in SARS-CoV-2 spike protein with angiotensin-converting enzyme 2.Sci Rep. 2021 Mar 25;11(1):6927. doi: 10.1038/s41598-021-86380-2. Sci Rep. 2021. PMID: 33767306 Free PMC article.
-
Different compounds against Angiotensin-Converting Enzyme 2 (ACE2) receptor potentially containing the infectivity of SARS-CoV-2: an in silico study.J Mol Model. 2022 Mar 5;28(4):82. doi: 10.1007/s00894-022-05059-1. J Mol Model. 2022. PMID: 35249180 Free PMC article.
-
Withanone from Withania somnifera Attenuates SARS-CoV-2 RBD and Host ACE2 Interactions to Rescue Spike Protein Induced Pathologies in Humanized Zebrafish Model.Drug Des Devel Ther. 2021 Mar 11;15:1111-1133. doi: 10.2147/DDDT.S292805. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33737804 Free PMC article.
-
Potential therapeutic approaches for the early entry of SARS-CoV-2 by interrupting the interaction between the spike protein on SARS-CoV-2 and angiotensin-converting enzyme 2 (ACE2).Biochem Pharmacol. 2021 Oct;192:114724. doi: 10.1016/j.bcp.2021.114724. Epub 2021 Aug 8. Biochem Pharmacol. 2021. PMID: 34371003 Free PMC article. Review.
-
Interactions of angiotensin-converting enzyme-2 (ACE2) and SARS-CoV-2 spike receptor-binding domain (RBD): a structural perspective.Mol Biol Rep. 2023 Mar;50(3):2713-2721. doi: 10.1007/s11033-022-08193-4. Epub 2022 Dec 23. Mol Biol Rep. 2023. PMID: 36562937 Free PMC article. Review.
Cited by
-
Absorption spectra of p-nitroaniline derivatives: charge transfer effects and the role of substituents.J Mol Model. 2024 Apr 2;30(5):120. doi: 10.1007/s00894-024-05917-0. J Mol Model. 2024. PMID: 38564015
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

