Cyclic Stretch-Induced Mechanical Stress Applied at 1 Hz Frequency Can Alter the Metastatic Potential Properties of SAOS-2 Osteosarcoma Cells
- PMID: 37175397
- PMCID: PMC10178551
- DOI: 10.3390/ijms24097686
Cyclic Stretch-Induced Mechanical Stress Applied at 1 Hz Frequency Can Alter the Metastatic Potential Properties of SAOS-2 Osteosarcoma Cells
Abstract
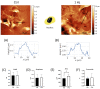
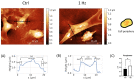
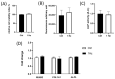
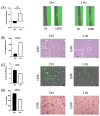
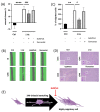
Recently, there has been an increasing focus on cellular morphology and mechanical behavior in order to gain a better understanding of the modulation of cell malignancy. This study used uniaxial-stretching technology to select a mechanical regimen able to elevate SAOS-2 cell migration, which is crucial in osteosarcoma cell pathology. Using confocal and atomic force microscopy, we demonstrated that a 24 h 0.5% cyclic elongation applied at 1 Hz induces morphological changes in cells. Following mechanical stimulation, the cell area enlarged, developing a more elongated shape, which disrupted the initial nuclear-to-cytoplasm ratio. The peripheral cell surface also increased its roughness. Cell-based biochemical assays and real-time PCR quantification showed that these morphologically induced changes are unrelated to the osteoblastic differentiative grade. Interestingly, two essential cell-motility properties in the modulation of the metastatic process changed following the 24 h 1 Hz mechanical stimulation. These were cell adhesion and cell migration, which, in fact, were dampened and enhanced, respectively. Notably, our results showed that the stretch-induced up-regulation of cell motility occurs through a mechanism that does not depend on matrix metalloproteinase (MMP) activity, while the inhibition of ion-stretch channels could counteract it. Overall, our results suggest that further research on mechanobiology could represent an alternative approach for the identification of novel molecular targets of osteosarcoma cell malignancy.
Keywords: biomechanical response; cell migration; cell morphology; cell structural deformation; cyclic mechanical strain; malignant bone cells; mechanical stress; mechano-sensing; mechanobiology; osteosarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

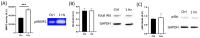
References
-
- Northey J.J., Przybyla L., Weaver V.M. Tissue Force Programs Cell Fate and Tumor Aggression. Cancer Discov. 2017;7:1224–1237. doi: 10.1158/2159-8290.CD-16-0733. - DOI - PMC - PubMed
-
- Jagodzinski M., Drescher M., Zeichen J., Hankemeier S., Krettek C., Bosch U., van Griensven M. Effects of Cyclic Longitudinal Mechanical Strain and Dexamethasone on Osteogenic Differentiation of Human Bone Marrow Stromal Cells. Eur. Cell. Mater. 2004;7:35–41. doi: 10.22203/eCM.v007a04. discussion 41. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

