An Evaluation of the Cytotoxic and Genotoxic Effects of the Marine Toxin C17-SAMT in Human TK6 and HepaRG Cell Lines
- PMID: 37175512
- PMCID: PMC10177896
- DOI: 10.3390/ijms24097805
An Evaluation of the Cytotoxic and Genotoxic Effects of the Marine Toxin C17-SAMT in Human TK6 and HepaRG Cell Lines
Abstract
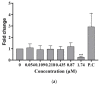
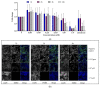
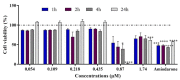
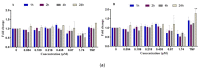
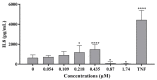
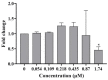
This study investigates the genotoxicity and cytotoxicity of C17-sphinganine analog mycotoxin (C17-SAMT) using in vitro assays. C17-SAMT was previously identified as the cause of unusual toxicity in cultured mussels from the Bizerte Lagoon in northern Tunisia. While a previous in vivo genotoxicity study was inconclusive, in vitro results demonstrated that C17-SAMT induced an increase in micronucleus formation in human lymphoblastoid TK6 cells at concentrations of 0.87 µM and 1.74 µM. In addition, multiparametric cytotoxicity assays were performed in the human hepatoma HepaRG cell line, which showed that C17-SAMT induced mitochondrial dysfunction, decreased cellular ATP levels, and altered the expression of various proteins, including superoxide dismutase SOD2, heme oxygenase HO-1, and NF-κB. These results suggest that C17-SAMT is mutagenic in vitro and can induce mitochondrial dysfunction in HepaRG cells. However, the exact mode of action of this toxin requires further investigation. Overall, this study highlights the potential toxicity of C17-SAMT and the need for further research to better understand its effects.
Keywords: C17-SAMT; in vitro; marine toxins; micronucleus assay; mitochondrial dysfunction; oxidative stress; pH3 phospho S10; γH2AX.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures







Similar articles
-
Investigation of the Genotoxic Potential of the Marine Toxin C17-SAMT Using the In Vivo Comet and Micronucleus Assays.Mar Drugs. 2022 Sep 30;20(10):619. doi: 10.3390/md20100619. Mar Drugs. 2022. PMID: 36286443 Free PMC article.
-
Marine toxin C17-SAMT causes major structural damage to vital organs in mice following subchronic toxicity trials.Ecotoxicol Environ Saf. 2023 May;256:114887. doi: 10.1016/j.ecoenv.2023.114887. Epub 2023 Apr 10. Ecotoxicol Environ Saf. 2023. PMID: 37043947
-
Toxic c17-sphinganine analogue mycotoxin, contaminating tunisian mussels, causes flaccid paralysis in rodents.Mar Drugs. 2013 Nov 28;11(12):4724-40. doi: 10.3390/md11124724. Mar Drugs. 2013. PMID: 24287956 Free PMC article.
-
Genotoxicity and potential carcinogenicity of cyanobacterial toxins - a review.Mutat Res. 2011 Jan-Apr;727(1-2):16-41. doi: 10.1016/j.mrrev.2011.01.002. Epub 2011 Jan 26. Mutat Res. 2011. PMID: 21277993 Review.
-
Does the recommended lymphocyte cytokinesis-block micronucleus assay for human biomonitoring actually detect DNA damage induced by occupational and environmental exposure to genotoxic chemicals?Mutagenesis. 2013 Jul;28(4):375-80. doi: 10.1093/mutage/get026. Epub 2013 May 3. Mutagenesis. 2013. PMID: 23644166 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

