Anti-Cancer Effects of Artesunate in Human 3D Tumor Models of Different Complexity
- PMID: 37175551
- PMCID: PMC10178545
- DOI: 10.3390/ijms24097844
Anti-Cancer Effects of Artesunate in Human 3D Tumor Models of Different Complexity
Abstract
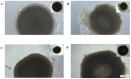
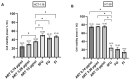
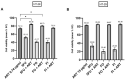
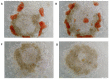
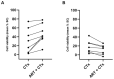
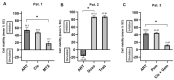
The anti-malaria drug Artesunate (ART) shows strong anti-cancer effects in vitro; however, it shows only marginal treatment results in clinical cancer studies. In this study, ART was tested in preclinical 3D cancer models of increasing complexity using clinically relevant peak plasma concentrations to obtain further information for translation into clinical use. ART reduced cell viability in HCT-116 and HT-29 derived cancer spheroids (p < 0.001). HCT-116 spheroids responded dose-dependently, while HT-29 spheroids were affected more strongly by ART than by cytostatics (p < 0.001). HCT-116 spheroids were chemo-sensitized by ART (p < 0.001). In patient-derived cancer spheroids (PDCS), ART led to inhibition of cell viability in 84.62% of the 39 samples tested, with a mean inhibitory effect of 13.87%. Viability reduction of ART was 2-fold weaker than cytostatic monotherapies (p = 0.028). Meanwhile, tumor-stimulation of up to 16.30% was observed in six (15.38%) PDCS-models. In 15 PDCS samples, ART modulated chemotherapies in combined testing, eight of which showed chemo-stimulation (maximum of 36.90%) and seven chemo-inhibition (up to 16.95%). These results demonstrate that ART's anti-cancer efficacy depends on the complexity of the tumor model used. This emphasizes that cancer treatment with ART should be evaluated before treatment of the individual patient to ensure its benefits and prevent unwanted effects.
Keywords: 3D cancer model; Artesunate; artemisinin-derivatives; cancer spheroid model; neoplasia.
Conflict of interest statement
B.M. is co-founder of the SpheroTec GmbH. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
High-level artemisinin-resistance with quinine co-resistance emerges in P. falciparum malaria under in vivo artesunate pressure.BMC Med. 2018 Oct 1;16(1):181. doi: 10.1186/s12916-018-1156-x. BMC Med. 2018. PMID: 30269689 Free PMC article.
-
The anti-malarial artesunate is also active against cancer.Int J Oncol. 2001 Apr;18(4):767-73. doi: 10.3892/ijo.18.4.767. Int J Oncol. 2001. PMID: 11251172
-
The anti-malarial drug artesunate causes cell cycle arrest and apoptosis of triple-negative MDA-MB-468 and HER2-enriched SK-BR-3 breast cancer cells.Exp Mol Pathol. 2019 Apr;107:10-22. doi: 10.1016/j.yexmp.2019.01.006. Epub 2019 Jan 17. Exp Mol Pathol. 2019. PMID: 30660598
-
[Combined antimalarial therapy using artemisinin].Parassitologia. 2004 Jun;46(1-2):85-7. Parassitologia. 2004. PMID: 15305693 Review. Italian.
-
Enhancing the antimalarial activity of artesunate.Parasitol Res. 2020 Sep;119(9):2749-2764. doi: 10.1007/s00436-020-06786-1. Epub 2020 Jul 7. Parasitol Res. 2020. PMID: 32638101 Free PMC article. Review.
Cited by
-
Artemisinin's molecular symphony: illuminating pathways for cancer therapy.Mol Biol Rep. 2024 Dec 31;52(1):95. doi: 10.1007/s11033-024-10202-7. Mol Biol Rep. 2024. PMID: 39739138 Review.
-
Anti-Cancer Properties of Two Intravenously Administrable Curcumin Formulations as Evaluated in the 3D Patient-Derived Cancer Spheroid Model.Int J Mol Sci. 2024 Aug 5;25(15):8543. doi: 10.3390/ijms25158543. Int J Mol Sci. 2024. PMID: 39126111 Free PMC article.
-
Novel artesunate-metformin conjugate inhibits bladder cancer cell growth associated with Clusterin/SREBP1/FASN signaling pathway.Korean J Physiol Pharmacol. 2024 May 1;28(3):219-227. doi: 10.4196/kjpp.2024.28.3.219. Korean J Physiol Pharmacol. 2024. PMID: 38682170 Free PMC article.
References
-
- Wells J.C., Sidhu A., Ding K., Smoragiewicz M., Heng D.Y.C., Shepherd F.A., Ellis P.M., Bradbury P.A., Jonker D.J., Siu L.L., et al. Complementary Medicine Use Amongst Patients with Metastatic Cancer Enrolled in Phase III Clinical Trials. Oncologist. 2022;27:e286–e293. doi: 10.1093/oncolo/oyac020. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

