Oligomeric State and Holding Activity of Hsp60
- PMID: 37175554
- PMCID: PMC10177986
- DOI: 10.3390/ijms24097847
Oligomeric State and Holding Activity of Hsp60
Abstract
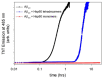
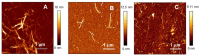
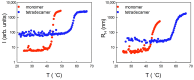
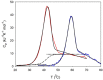
Similar to its bacterial homolog GroEL, Hsp60 in oligomeric conformation is known to work as a folding machine, with the assistance of co-chaperonin Hsp10 and ATP. However, recent results have evidenced that Hsp60 can stabilize aggregation-prone molecules in the absence of Hsp10 and ATP by a different, "holding-like" mechanism. Here, we investigated the relationship between the oligomeric conformation of Hsp60 and its ability to inhibit fibrillization of the Ab40 peptide. The monomeric or tetradecameric form of the protein was isolated, and its effect on beta-amyloid aggregation was separately tested. The structural stability of the two forms of Hsp60 was also investigated using differential scanning calorimetry (DSC), light scattering, and circular dichroism. The results showed that the protein in monomeric form is less stable, but more effective against amyloid fibrillization. This greater functionality is attributed to the disordered nature of the domains involved in subunit contacts.
Keywords: Hsp60; amyloid aggregation; monomer; non-canonical function; oligomer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The human mitochondrial Hsp60 in the APO conformation forms a stable tetradecameric complex.Cell Cycle. 2017 Jul 3;16(13):1309-1319. doi: 10.1080/15384101.2017.1321180. Epub 2017 Jun 8. Cell Cycle. 2017. PMID: 28594255 Free PMC article.
-
Physicochemical Properties of the Mammalian Molecular Chaperone HSP60.Int J Mol Sci. 2018 Feb 6;19(2):489. doi: 10.3390/ijms19020489. Int J Mol Sci. 2018. PMID: 29415503 Free PMC article.
-
Significance of chaperonin 10-mediated inhibition of ATP hydrolysis by chaperonin 60.Proc Natl Acad Sci U S A. 1997 Aug 19;94(17):9011-6. doi: 10.1073/pnas.94.17.9011. Proc Natl Acad Sci U S A. 1997. PMID: 9256426 Free PMC article.
-
Structure and function in GroEL-mediated protein folding.Annu Rev Biochem. 1998;67:581-608. doi: 10.1146/annurev.biochem.67.1.581. Annu Rev Biochem. 1998. PMID: 9759498 Review.
-
Reaction Cycle of Chaperonin GroEL via Symmetric "Football" Intermediate.J Mol Biol. 2015 Sep 11;427(18):2912-8. doi: 10.1016/j.jmb.2015.04.007. Epub 2015 Apr 18. J Mol Biol. 2015. PMID: 25900372 Review.
Cited by
-
Targeting PRMT3 impairs methylation and oligomerization of HSP60 to boost anti-tumor immunity by activating cGAS/STING signaling.Nat Commun. 2024 Sep 10;15(1):7930. doi: 10.1038/s41467-024-52170-3. Nat Commun. 2024. PMID: 39256398 Free PMC article.
-
Molecular Chaperonin HSP60: Current Understanding and Future Prospects.Int J Mol Sci. 2024 May 17;25(10):5483. doi: 10.3390/ijms25105483. Int J Mol Sci. 2024. PMID: 38791521 Free PMC article. Review.
-
The Role of Helicobacter pylori Heat Shock Proteins in Gastric Diseases' Pathogenesis.Int J Mol Sci. 2025 May 24;26(11):5065. doi: 10.3390/ijms26115065. Int J Mol Sci. 2025. PMID: 40507876 Free PMC article. Review.
-
The Ability of DNAJB6b to Suppress Amyloid Formation Depends on the Chaperone Aggregation State.ACS Chem Neurosci. 2024 May 1;15(9):1732-1737. doi: 10.1021/acschemneuro.4c00120. Epub 2024 Apr 19. ACS Chem Neurosci. 2024. PMID: 38640082 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

