Hypoxic Stress-Dependent Regulation of Na,K-ATPase in Ischemic Heart Disease
- PMID: 37175562
- PMCID: PMC10177966
- DOI: 10.3390/ijms24097855
Hypoxic Stress-Dependent Regulation of Na,K-ATPase in Ischemic Heart Disease
Abstract
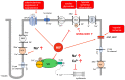
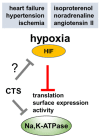
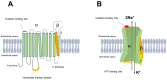
In cardiomyocytes, regular activity of the Na,K-ATPase (NKA) and its Na/K pump activity is essential for maintaining ion gradients, excitability, propagation of action potentials, electro-mechanical coupling, trans-membrane Na+ and Ca2+ gradients and, thus, contractility. The activity of NKA is impaired in ischemic heart disease and heart failure, which has been attributed to decreased expression of the NKA subunits. Decreased NKA activity leads to intracellular Na+ and Ca2+ overload, diastolic dysfunction and arrhythmias. One signal likely related to these events is hypoxia, where hypoxia-inducible factors (HIF) play a critical role in the adaptation of cells to low oxygen tension. HIF activity increases in ischemic heart, hypertension, heart failure and cardiac fibrosis; thus, it might contribute to the impaired function of NKA. This review will mainly focus on the regulation of NKA in ischemic heart disease in the context of stressed myocardium and the hypoxia-HIF axis and argue on possible consequences of treatment.
Keywords: HIF; Na,K-ATPase; cardiotonic steroids; cellular stress; heart failure; hypoxia; ion transporter; ischemic heart.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Role of Na+-K+ ATPase Alterations in the Development of Heart Failure.Int J Mol Sci. 2024 Oct 8;25(19):10807. doi: 10.3390/ijms251910807. Int J Mol Sci. 2024. PMID: 39409137 Free PMC article. Review.
-
Hypokalaemia induces Ca²⁺ overload and Ca²⁺ waves in ventricular myocytes by reducing Na⁺,K⁺-ATPase α₂ activity.J Physiol. 2015 Mar 15;593(6):1509-21. doi: 10.1113/jphysiol.2014.279893. Epub 2014 Nov 11. J Physiol. 2015. PMID: 25772299 Free PMC article.
-
Activating Na+-K+ ATPase: a potential cardioprotective therapy during early hemorrhagic shock.Med Hypotheses. 2014 Dec;83(6):685-7. doi: 10.1016/j.mehy.2014.09.018. Epub 2014 Oct 13. Med Hypotheses. 2014. PMID: 25459134
-
HIF-2α Controls Expression and Intracellular Trafficking of the α2-Subunit of Na,K-ATPase in Hypoxic H9c2 Cardiomyocytes.Biomedicines. 2023 Oct 24;11(11):2879. doi: 10.3390/biomedicines11112879. Biomedicines. 2023. PMID: 38001879 Free PMC article.
-
Na⁺ transport in the normal and failing heart - remember the balance.J Mol Cell Cardiol. 2013 Aug;61:2-10. doi: 10.1016/j.yjmcc.2013.04.011. Epub 2013 Apr 19. J Mol Cell Cardiol. 2013. PMID: 23608603 Free PMC article. Review.
Cited by
-
Ferroptosis: Emerging Role in Diseases and Potential Implication of Bioactive Compounds.Int J Mol Sci. 2023 Dec 8;24(24):17279. doi: 10.3390/ijms242417279. Int J Mol Sci. 2023. PMID: 38139106 Free PMC article. Review.
-
Myocardial Revascularization in Patients with Diabetes and Heart Failure-A Narrative Review.Int J Mol Sci. 2025 Apr 5;26(7):3398. doi: 10.3390/ijms26073398. Int J Mol Sci. 2025. PMID: 40244271 Free PMC article. Review.
-
Mechanisms mediating effects of cardiotonic steroids in mammalian blood cells.Front Pharmacol. 2025 Mar 24;16:1520927. doi: 10.3389/fphar.2025.1520927. eCollection 2025. Front Pharmacol. 2025. PMID: 40196366 Free PMC article. Review.
-
Enhanced lipid metabolism reprogramming in CHF rats through IL-6-mediated cardiac glial cell modulation by digilanid C and electroacupuncture stimulation combination.Front Cell Dev Biol. 2024 Sep 3;12:1424395. doi: 10.3389/fcell.2024.1424395. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39291267 Free PMC article.
-
The Role of Ion-Transporting Proteins in Human Disease.Int J Mol Sci. 2024 Jan 31;25(3):1726. doi: 10.3390/ijms25031726. Int J Mol Sci. 2024. PMID: 38339004 Free PMC article.
References
-
- Schatzmann H.J. Cardiac glycosides as inhibitors of active potassium and sodium transport by erythrocyte membrane. Helv. Physiol. Pharmacol. Acta. 1953;11:346–354. - PubMed
-
- Drummond C.A., Hill M.C., Shi H., Fan X., Xie J.X., Haller S.T., Kennedy D.J., Liu J., Garrett M.R., Xie Z., et al. Na/K−ATPase signaling regulates collagen synthesis through microRNA-29b-3p in cardiac fibroblasts. Physiol. Genom. 2016;48:220–229. doi: 10.1152/physiolgenomics.00116.2015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

