Estrogenic Modulation of Ionic Channels, Pumps and Exchangers in Airway Smooth Muscle
- PMID: 37175587
- PMCID: PMC10178541
- DOI: 10.3390/ijms24097879
Estrogenic Modulation of Ionic Channels, Pumps and Exchangers in Airway Smooth Muscle
Abstract
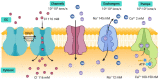
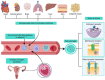
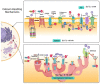
To preserve ionic homeostasis (primarily Ca2+, K+, Na+, and Cl-), in the airway smooth muscle (ASM) numerous transporters (channels, exchangers, and pumps) regulate the influx and efflux of these ions. Many of intracellular processes depend on continuous ionic permeation, including exocytosis, contraction, metabolism, transcription, fecundation, proliferation, and apoptosis. These mechanisms are precisely regulated, for instance, through hormonal activity. The lipophilic nature of steroidal hormones allows their free transit into the cell where, in most cases, they occupy their cognate receptor to generate genomic actions. In the sense, estrogens can stimulate development, proliferation, migration, and survival of target cells, including in lung physiology. Non-genomic actions on the other hand do not imply estrogen's intracellular receptor occupation, nor do they initiate transcription and are mostly immediate to the stimulus. Among estrogen's non genomic responses regulation of calcium homeostasis and contraction and relaxation processes play paramount roles in ASM. On the other hand, disruption of calcium homeostasis has been closely associated with some ASM pathological mechanism. Thus, this paper intends to summarize the effects of estrogen on ionic handling proteins in ASM. The considerable diversity, range and power of estrogens regulates ionic homeostasis through genomic and non-genomic mechanisms.
Keywords: airway smooth muscle; channels; estrogen; exchangers; pumps; receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Ionic mechanisms and Ca(2+) regulation in airway smooth muscle contraction: do the data contradict dogma?Am J Physiol Lung Cell Mol Physiol. 2002 Jun;282(6):L1161-78. doi: 10.1152/ajplung.00452.2001. Am J Physiol Lung Cell Mol Physiol. 2002. PMID: 12003770 Review.
-
Research progress in the mechanism of calcium ion on contraction and relaxation of airway smooth muscle cells.J Recept Signal Transduct Res. 2021 Apr;41(2):117-122. doi: 10.1080/10799893.2020.1806315. Epub 2020 Aug 18. J Recept Signal Transduct Res. 2021. PMID: 32808844 Review.
-
The short-chain free fatty acid receptor FFAR3 is expressed and potentiates contraction in human airway smooth muscle.Am J Physiol Lung Cell Mol Physiol. 2020 Jun 1;318(6):L1248-L1260. doi: 10.1152/ajplung.00357.2019. Epub 2020 Mar 25. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32209026 Free PMC article.
-
TRPC3 regulates release of brain-derived neurotrophic factor from human airway smooth muscle.Biochim Biophys Acta. 2013 Dec;1833(12):2953-2960. doi: 10.1016/j.bbamcr.2013.07.019. Epub 2013 Jul 27. Biochim Biophys Acta. 2013. PMID: 23899746 Free PMC article.
-
Regulation of second messengers associated with airway smooth muscle contraction and relaxation.Am J Respir Crit Care Med. 1998 Nov;158(5 Pt 3):S115-22. doi: 10.1164/ajrccm.158.supplement_2.13tac700. Am J Respir Crit Care Med. 1998. PMID: 9817734 Review.
Cited by
-
Electrically conductive biopolymer-based hydrogels and fibrous materials fabricated using 3D printing and electrospinning for cardiac tissue engineering.Bioact Mater. 2025 Jun 9;51:650-719. doi: 10.1016/j.bioactmat.2025.05.014. eCollection 2025 Sep. Bioact Mater. 2025. PMID: 40546729 Free PMC article. Review.
-
Nuclear receptors in health and disease: signaling pathways, biological functions and pharmaceutical interventions.Signal Transduct Target Ther. 2025 Jul 28;10(1):228. doi: 10.1038/s41392-025-02270-3. Signal Transduct Target Ther. 2025. PMID: 40717128 Free PMC article. Review.
-
Sex differences in airway disease: estrogen and airway surface liquid dynamics.Biol Sex Differ. 2024 Jul 18;15(1):56. doi: 10.1186/s13293-024-00633-z. Biol Sex Differ. 2024. PMID: 39026347 Free PMC article. Review.
-
Analysis of global gene expression using RNA-sequencing reveals novel mechanism of Yanghe Pingchuan decoction in the treatment of asthma.BMC Pulm Med. 2024 Mar 18;24(1):137. doi: 10.1186/s12890-024-02952-8. BMC Pulm Med. 2024. PMID: 38500104 Free PMC article.
-
The Mechanism by Which Estrogen Level Affects Knee Osteoarthritis Pain in Perimenopause and Non-Pharmacological Measures.Int J Mol Sci. 2025 Mar 7;26(6):2391. doi: 10.3390/ijms26062391. Int J Mol Sci. 2025. PMID: 40141035 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

