Molecular Mechanisms of Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury
- PMID: 37175592
- PMCID: PMC10177842
- DOI: 10.3390/ijms24097885
Molecular Mechanisms of Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury
Abstract
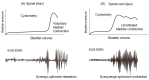
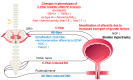
This article provides a synopsis of current progress made in fundamental studies of lower urinary tract dysfunction (LUTD) after spinal cord injury (SCI) above the sacral level. Animal models of SCI allowed us to examine the effects of SCI on the micturition control and the underlying neurophysiological processes of SCI-induced LUTD. Urine storage and elimination are the two primary functions of the LUT, which are governed by complicated regulatory mechanisms in the central and peripheral nervous systems. These neural systems control the action of two functional units in the LUT: the urinary bladder and an outlet consisting of the bladder neck, urethral sphincters, and pelvic-floor striated muscles. During the storage phase, the outlet is closed, and the bladder is inactive to maintain a low intravenous pressure and continence. In contrast, during the voiding phase, the outlet relaxes, and the bladder contracts to facilitate adequate urine flow and bladder emptying. SCI disrupts the normal reflex circuits that regulate co-ordinated bladder and urethral sphincter function, leading to involuntary and inefficient voiding. Following SCI, a spinal micturition reflex pathway develops to induce an overactive bladder condition following the initial areflexic phase. In addition, without proper bladder-urethral-sphincter coordination after SCI, the bladder is not emptied as effectively as in the normal condition. Previous studies using animal models of SCI have shown that hyperexcitability of C-fiber bladder afferent pathways is a fundamental pathophysiological mechanism, inducing neurogenic LUTD, especially detrusor overactivity during the storage phase. SCI also induces neurogenic LUTD during the voiding phase, known as detrusor sphincter dyssynergia, likely due to hyperexcitability of Aδ-fiber bladder afferent pathways rather than C-fiber afferents. The molecular mechanisms underlying SCI-induced LUTD are multifactorial; previous studies have identified significant changes in the expression of various molecules in the peripheral organs and afferent nerves projecting to the spinal cord, including growth factors, ion channels, receptors and neurotransmitters. These findings in animal models of SCI and neurogenic LUTD should increase our understanding of pathophysiological mechanisms of LUTD after SCI for the future development of novel therapies for SCI patients with LUTD.
Keywords: Aδ-fiber afferent; C-fiber afferent; detrusor overactivity; detrusor–sphincter dyssynergia; fibrosis; spinal-cord injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

