Mechanisms of Oxidative Stress in Metabolic Syndrome
- PMID: 37175603
- PMCID: PMC10178199
- DOI: 10.3390/ijms24097898
Mechanisms of Oxidative Stress in Metabolic Syndrome
Abstract
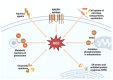
Metabolic syndrome is a cluster of conditions associated with the risk of diabetes mellitus type 2 and cardiovascular diseases (CVDs). Metabolic syndrome is closely related to obesity. Increased adiposity promotes inflammation and oxidative stress, which are precursors of various complications involving metabolic syndrome components, namely insulin resistance, hypertension, and hyperlipidemia. An increasing number of studies confirm the importance of oxidative stress and chronic inflammation in the etiology of metabolic syndrome. However, few studies have reviewed the mechanisms underlying the role of oxidative stress in contributing to metabolic syndrome. In this review, we highlight mechanisms by which reactive oxygen species (ROS) increase mitochondrial dysfunction, protein damage, lipid peroxidation, and impair antioxidant function in metabolic syndrome. Biomarkers of oxidative stress can be used in disease diagnosis and evaluation of severity.
Keywords: cardiovascular disease; hyperglycemia; hyperlipidemia; hypertension; inflammatory cytokines; insulin resistance; metabolic syndrome; obesity; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Gallardo-Alfaro L., Bibiloni M.d.M., Mascaró C.M., Montemayor S., Ruiz-Canela M., Salas-Salvadó J., Corella D., Fitó M., Romaguera D., Vioque J., et al. Leisure-Time Physical Activity, Sedentary Behaviour and Diet Quality Are Associated with Metabolic Syndrome Severity: The PREDIMED-Plus Study. Nutrients. 2020;12:1013. doi: 10.3390/nu12041013. - DOI - PMC - PubMed
-
- Wilkinson M.J., Manoogian E.N.C., Zadourian A., Lo H., Fakhouri S., Shoghi A., Wang X., Fleischer J.G., Navlakha S., Panda S., et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020;31:92–104.e5. doi: 10.1016/j.cmet.2019.11.004. - DOI - PMC - PubMed
-
- Dieli-Conwright C.M., Courneya K.S., Demark-Wahnefried W., Sami N., Lee K., Buchanan T.A., Spicer D.V., Tripathy D., Bernstein L., Mortimer J.E. Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018;36:875–883. doi: 10.1200/JCO.2017.75.7526. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

